Translate this page into:
An evaluation and correlation of airway space of pharynx, mandibular morphology, and tongue volume in skeletal classes and facial patterns – A cone beam computed tomography study

*Corresponding author: Dr Safiya Sana, Professor and Head, Department of Orthodontics, ESIC Dental College, Kalaburagi, Karnataka, India. sanommi_123@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Sana S, Patil R, Jain P, Kondody RT, Gaikwad S. An evaluation and correlation of airway space of pharynx, mandibular morphology, and tongue volume in skeletal classes and facial patterns – A cone beam computed tomography study. APOS Trends Orthod. doi: 10.25259/APOS_122_2024
Abstract
Objectives:
Respiration and its function have a direct relationship with the pharyngeal airway, mandibular morphology, and tongue. The objective of this study was to evaluate and correlate pharyngeal airway space, mandibular morphology, and tongue volume in various skeletal classes and facial patterns.
Material and Methods:
A total of 120 pre-treatment cone beam computed tomography (CBCT) images were randomly classified into 3 skeletal classes (40/group). Each class was further categorized into hyperdivergent and hypodivergent growth patterns. Linear and angular measurements were estimated using three-dimensional digital imaging programs (Kavo 3D OnDemand software), and pharyngeal airway and tongue space were volumetrically analyzed by ITK-SNAP segmentation software.
Results:
All the measured variables showed highly significant differences except for the anterior-posterior angle of the mandible, which was statistically insignificant with P = 0.675. The simple regression was formulated to assess the volume of airway space.
Conclusion:
Hyperdivergent subjects had reduced pharyngeal airway space and tongue volume when compared to hypodivergent subjects. Among all the subgroups, Class III hypodivergent showed the highest pharyngeal airway volume and tongue volume, and the least was found in Class II hypodivergent. A direct relationship was estimated between airway mandibular morphology and tongue volume, recommending thorough analysis of oropharyngeal structures in a non-individualized way for orthodontic diagnosis and treatment planning.
Keywords
Airway
Cone beam computed tomography
Pharynx
Tongue
INTRODUCTION
The pharynx is a critical structure in the human respiratory system that not only has a significant role in deglutition and respiration but also has a crucial role in the growth and development of bones of craniofacial regions.[1,2] Any abnormalities in the soft tissue and craniofacial skeleton can change the pharyngeal airway system as a result of the posterior position of the mandible or maxillary retrognathism not only induces airway insufficiency and mouth breathing but also results in downward rotation of mandible, tongue, and extension of the head.[3,4] Furthermore, an extended head position and lowered tongue posture increase the mandibular load due to stretching of facial musculature, resulting in upright incisors and narrow arches, and predominantly, such features are seen in hyperdivergent growth patterns.[5,6]
According to “soft tissue stretching hypothesis”[7,8] stated that alterations in the normal naso-respiratory processes due to stretching of soft tissues oro-pharyngeal area have an extreme impact on the craniofacial development, especially during the period of active growth when a patient approaches for orthodontic treatment.[6,9,10]
Previous studies showed that growing individuals with Class II malocclusion had a constricted pharyngeal area, mainly of the oropharynx and hypopharynx, in comparison to Class I malocclusion.[1-3,10] Furthermore, skeletal movements during orthognathic surgeries do have an effect on the surrounding structures, such as maxillo-mandibular advancement procedures that result in movement of the posterior of the tongue, soft palate, hyoid bone, and frontal pharyngeal structures anteriorly. Similarly, any mandibular setback surgeries are also associated with the narrowing of the pharyngeal space. [11,12]
Recent advancements in the field of technologies and healthcare systems, like the use of cone-beam computed tomography (CBCT), have overtaken the previous 2-D methods or procedures. These CBCT multiplanar reconstructions (MPRs) and 3D evaluations provide data that are more accurate and reduce radiation dose.[13-15]
Considering the significance of determining the morphology of the pharyngeal airway in different facial skeletal patterns and its effect on treatment planning, this study was carried out to evaluate and correlate pharyngeal airway space, mandibular morphology, and tongue volume in individuals with various skeletal classes and facial patterns.
MATERIAL AND METHODS
Study design
A study was performed on 120 patients whose CBCT images were recruited from the Department of Orthodontics and Dentofacial Orthopaedics. The Ethical Clearance Board, Al-Badar Rural Dental College, Kalaburagi, has approved this study, with certificate number (No. IEC/2019-20/03). The study was conducted from a period between December 2019 and July 2021. With a 95% confidence level and a margin of error of ±5%, a sample size of 120 subjects was allowed in the study. The power of sample size was analyzed by the formula n = f (α/2, β) × 2 × σ2/(µ1−µ2)2 where µ1 and µ2 are mean outcomes and σ is the standard deviation allowed in the study.
Inclusion criteria
The individuals were selected based on the inclusion criteria, i.e., healthy individuals in an age range of 18–20 years before orthodontic treatment or orthognathic surgery. The samples were classified into 3 skeletal classes: Class I, Class II, and Class III (40/group). Each class was further subdivided into two subgroups (20/subgroup) as hyperdivergent and hypodivergent, respectively as shown in Flowchart 1 below.
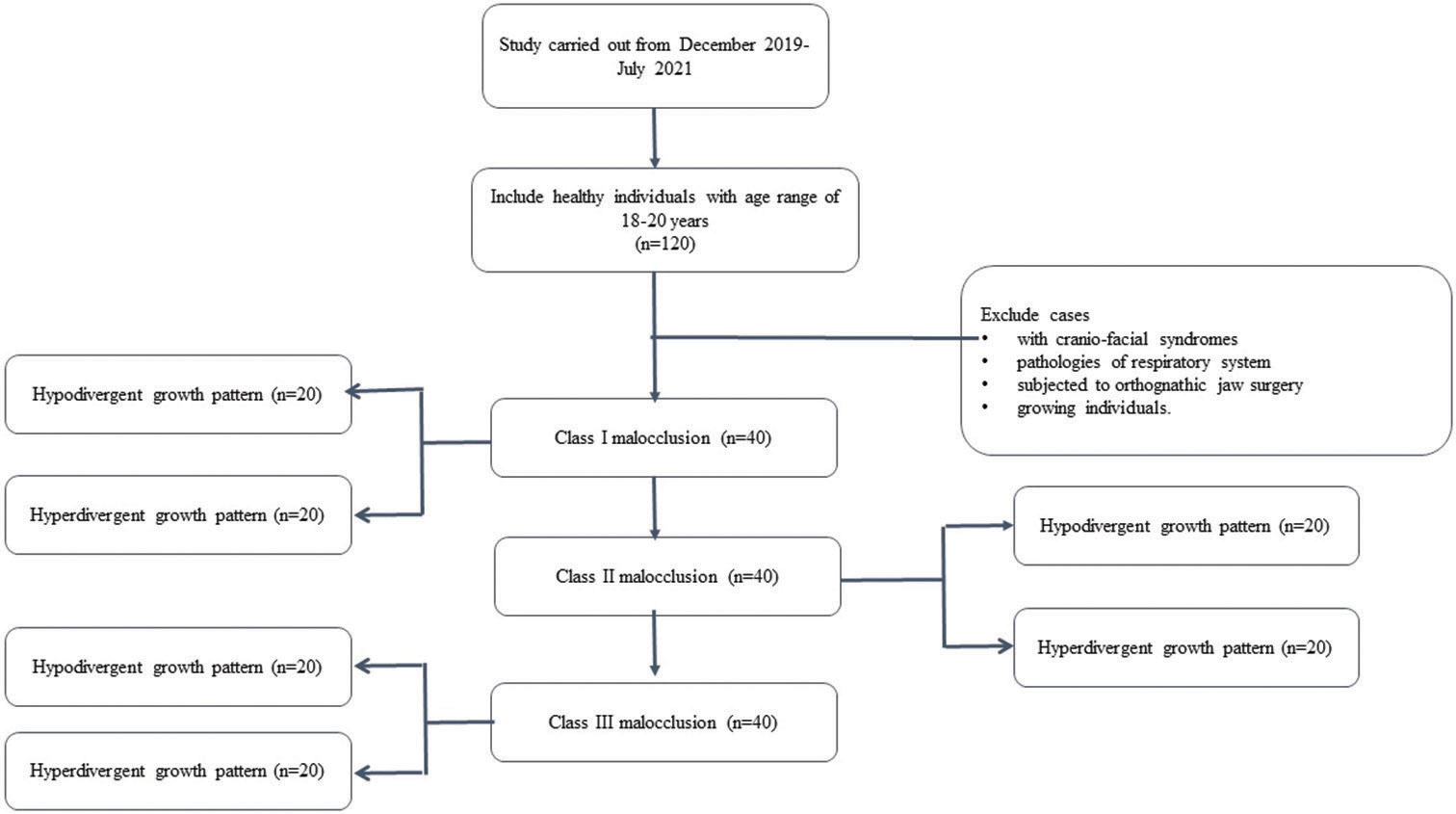
- Distribution of the samples.
Exclusion criteria
Individuals of growing age or younger than 18 years
Individuals with craniofacial syndromes
Individuals subjected to orthognathic surgery and pathologies of the respiratory system like the history of enlarged adenoids or ear-throat infections and obstructive sleep apnea (OSA) (exclusion of OSA patients was on the history of the presence of at least one of the symptoms such as snoring, daytime sleepiness, choking, and nocturnal awakening and a diagnostic criterion as described by the International Classification of Sleep Disorders for OSA)[16]
Procedure
CBCT volumes were captured (Kavo 3D Pro imaging system, Palodex group OY, Finland) at 120 kV, 10mA, field of view of 13 × 15 cm, voxel size of 0.4 mm, and scanning time of 40 s. The CBCT images were acquired with every individual seated in the upright position and with the eyeear plane parallel to the floor and the teeth of the patients in the maximal intercuspal position. The pre-treatment digital lateral cephalometric images were collected (CS IMAGING TECHNIQUE CS 8100 Rochester, NY USA). Digital Images (DICOM format) were exported to FACAD software (Version 3.10.1 Swedish Company, Ilexis AB Linkoping, SWEDEN) to classify into various classes and facial patterns.
The skeletal class was determined based on ANB, SNA, and SNB angle, while the facial pattern was differentiated as hypodivergent and hyperdivergent groups by calculating the VERT index of Rickets.[17]
The CBCT DICOM files were transferred to on-demand 3D software (version 1.0.10.746; CybeMed, Seoul, South Korea) for conversion to volumetric 3D MPR. For pharyngeal airway analysis, the reference line vertically was placed in the median sagittal plane, and the horizontal reference line was placed from the anterior nasal spine (ANS) to the posterior nasal spine (PNS) to reconstruct in axial and sagittal planes. Considering the mandible, the horizontal reference line was placed tangent to the lower border of the mandible for reconstruction in the sagittal plane and then extended to the genial tubercle in the superior direction [Figure 1a-d].[1]
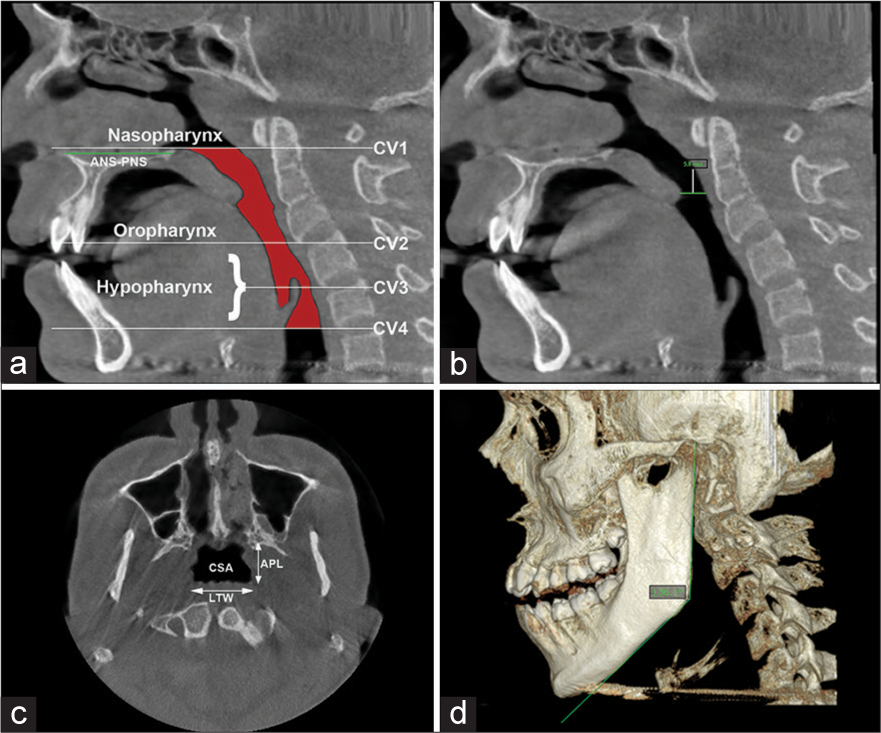
- Parameters for pharyngeal airway analysis (a) Determine the reference planes sagitally, as the distance from anterior nasal spine to posterior nasal spine as CV1 plane. Cv1 to CV2 as Oropharynx and CV2 to Cv4 as Hypopharynx; (b) Constricted distance; (c) largest transverse width and anteroposterior length determines the anatomic characteristics of the upper airway and cross-section area on axial plane. Largest transverse width, anteroposterior length; (d) Gonial angle of mandible.
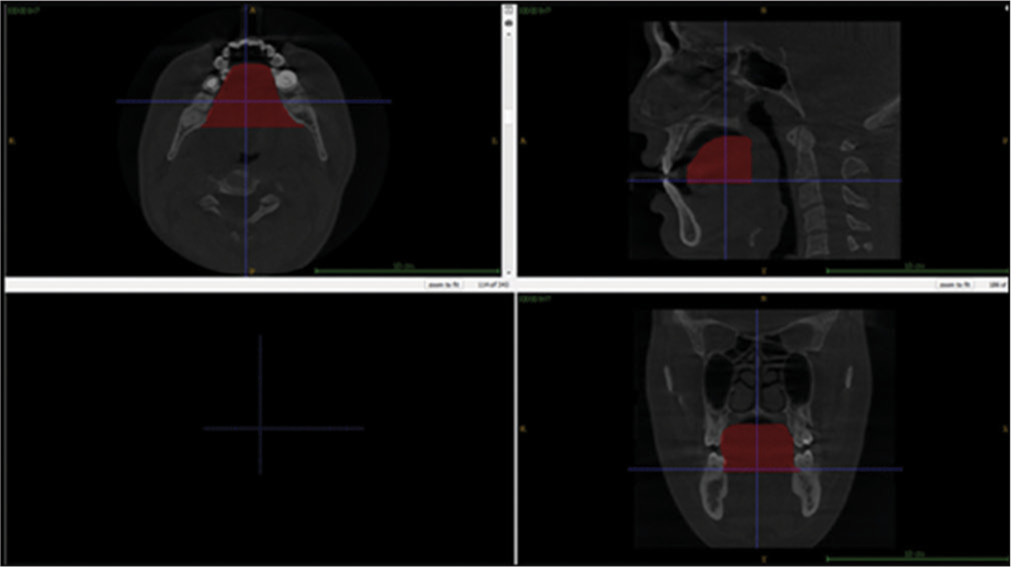
The analysis of the pharyngeal space volume (PSV) was accomplished from a 3D model. From the reconstructed 3D model, PVS was measured in mm3. PSV was evaluated by exporting the CBCT DICOM files to the ITK-SNAP software (version 3.8.0; Cognitica, Philadelphia, Pa; www.itksnap.org). From the MPR of images, the 3D models of the volume of the oropharynx and hypopharynx were reconstructed by utilizing the semi-automated segmented mode of the ITK-SNAP software.
The PSV in this research relates to the fusion between the oropharynx and the hypopharynx. Thus, we established the anatomic borderline according to Park et al.,[1,18] i.e., superiorly by taking a reference line at the right angle to midsagittal plane traced from the posterior-most point of the palatal plane (ANS-PNS) to the inferior most point of the C1 (first cervical vertebra) as CV1 plane and the anterior-inferior most point of the C2 (2nd cervical vertebra) as CV2 plane, similarly, CV3 plane and CV4 plane.[1] Based on these reference planes, the upper airway is divided into the oropharynx (between the CV1 and CV2 planes) and the hypopharynx (between the CV2 and CV4 planes) [Figure 1a]. Assessments of the anatomical parameters of the upper airway were measured as the largest transverse width, anteroposterior length, and cross-sectional area on the axial plane as shown in [Figures 1c, 2 and 3]. Parametres for measurement of mandibular morphology was shown in [Figure 4].
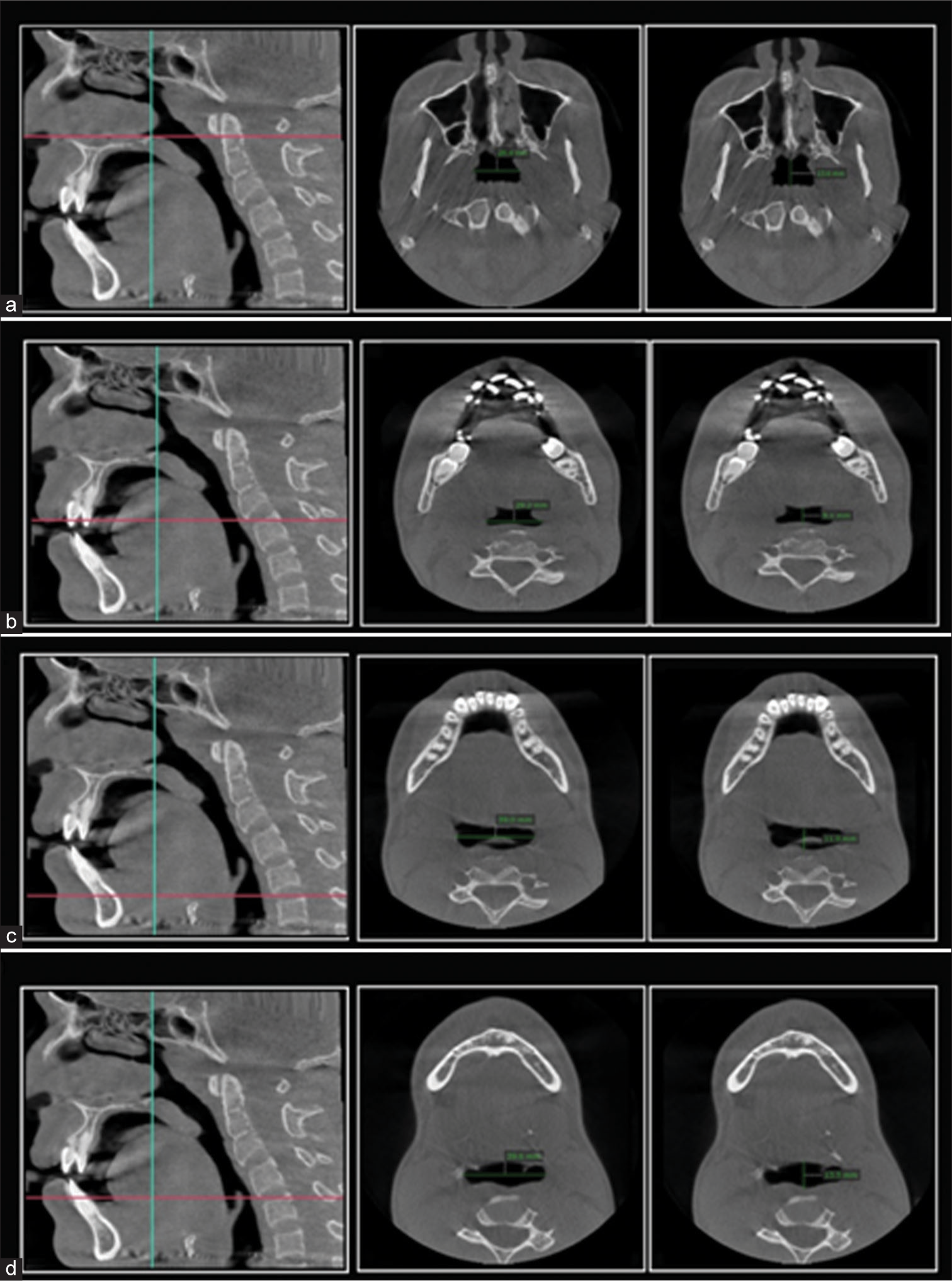
- Parameters for pharyngeal airway analysis at the level of cervical vertebrae and epiglottis (a) interspace at C1, interspace at C1 anterioposteriorly, (b) interspace at C2 and interspace at C2 anterioposterioly, (c) interspace at C3 and Interspace at C3 anterioposteriorly, (d) Interspace at epiglottis and Interspace at epiglottis anterioposteriorly
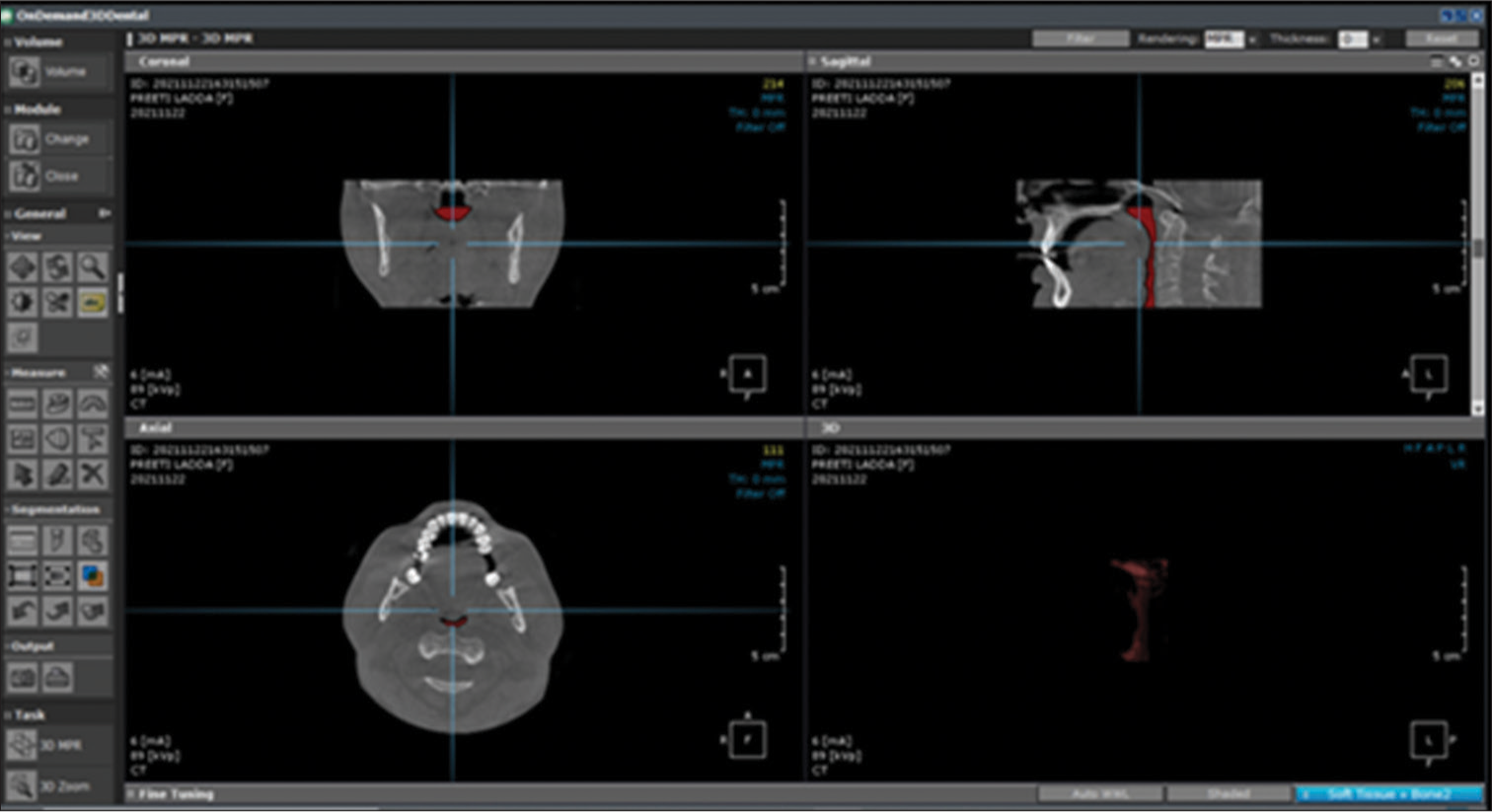
- Segmentation of pharyngeal space described in the red text represents the 3D volume of union between the oropharynx and the hypopharynx.
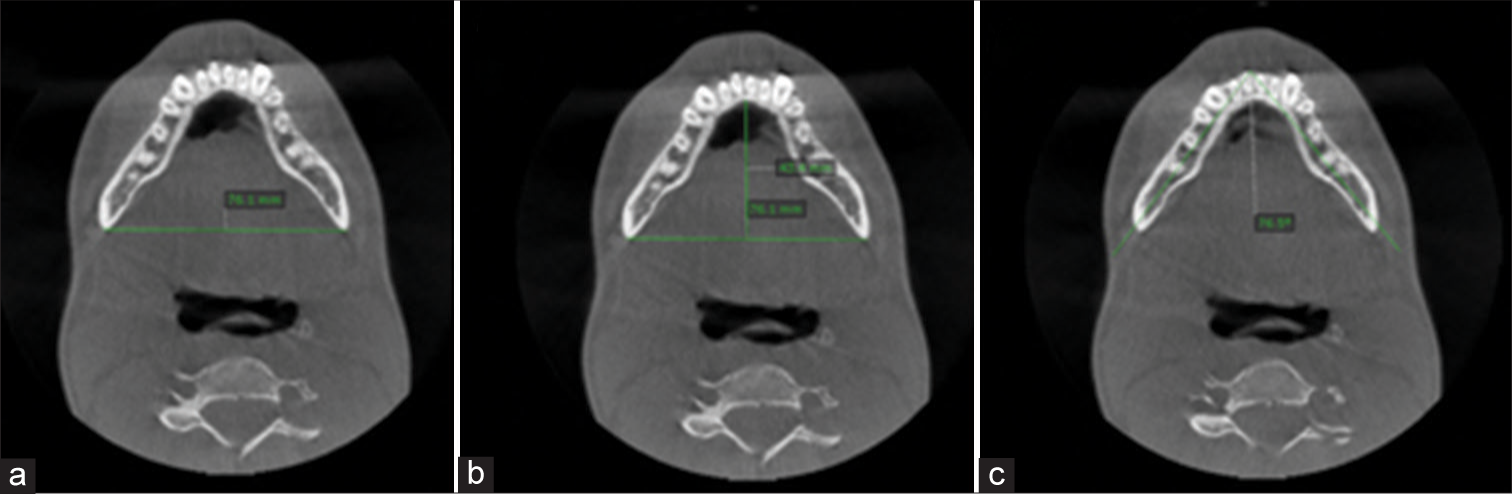
- Parameters for mandibular morphology analysis (a) Inter-distance of mandible; (b) anterior-posterior distance of mandible; (c) Transverse angle of mandible.
Boundaries to measure tongue volume were identified as the cementoenamel junction of posterior teeth (first molar and premolars) parallel to the X-axis plane both sagittally and axially, defining the ventral aspect of the tongue for segmentation. A plane at the right angle from the PNS in the axial orientation is defined to form the posterior aspect of the tongue for segmentation on the axial view. The occlusal plane was defined from the central cusps of the lower first molar to the incisal edge of the incisors for volume analysis, as shown in [Figure 5].
- Segmentation of the tongue described in red text represents the 3D volume of tongue.
As the tongue is a soft tissue, for each patient, Hounsfield values (−650–200 HU) were set to calculate the maximum amount of voxel. According to the Hounsfield values chosen initially, the above-defined borders for the tongue were then added to form a three-dimensional mask of the volume of the tongue.
Volumetric analysis of the tongue was determined by utilizing the voxel volume from the scan and the number of voxels taken for a specific mask. To rule out inter-investigator variance, the same investigator determined all the parameters 2 times with a 1-week interval. To determine the reproducibility of the measured parameters, the intra-class correlation coefficient was calculated.[19]
The parameters analyzed are represented in [Tables 1 and 2].
| Parameters | Definition of Parameters | Reconstructed plane |
|---|---|---|
| Pharyngeal space dimensions | ||
| ANS to PNS distance [Figure 1a] | Linear distance from the anterior most to posterior most point on the palatal plane. | Sagittal |
| Constricted distance [Figure 2b] | Linear distance from the narrowest area of pharyngeal space horizontally | Sagittal |
| Intrerspace at C1 (lateral to lateral [LLC1]) [Figure 2a] | Linear distance horizontally from the greatest lateral to lateral measurement of pharyngeal space situated at the lowermost point at C1 | Axial |
| Interspace at C1 anterioposteriorly (APC1)[Figure 2a] | Linear distance vertically from the greatest measurement anterioposteriorly of pharyngeal space situated at the lowermost point at C1 | Axial |
| Interspace at C2 (lateral to lateral [LLC2]) [Figure 2b] | Linear distance in horizontal direction from the greatest laterallateral measurement of pharyngeal space situated at the lowermost point at C2 | Axial |
| Interspace at C2 anterioposteriorly (APC2) [Figure 2b] | Linear distance vertically from the greatest measurement anterioposteriorly of pharyngeal space situated at the lowermost point at C2 | Axial |
| Interspace at C3 (lateral to lateral [LLC3]) [Figure 2c] | Linear distance horizontally from the greatest lateral to lateral measurement of pharyngeal space situated at the lowermost point at C3 | Axial |
| Interspace at C3 anterioposteriorly [APC3][Figure 2c] | Linear distance vertically from the greatest measurement anterioposteriorly of pharyngeal space situated at the lowermost point at C3 | Axial |
| Interspace at epiglottis (lateral to lateral [Epiglotiss-LL]) [Figure 2d] | Linear distance horizontally from the greatest lateral to lateral measurement of pharyngeal space situated at the point of maximum concavity of the stalk of the epiglottis. | Axial |
| Interspace at epiglottisanterioposteriorly (Epiglottis-AP) [Figure 2d] | Linear distance vertically from the greatest measurement anterioposteriorly of pharyngeal space situated at the point of maximum concavity of stalk of the epiglottis. | Axial |
ANS: Anterior nasal spine, PNS: Posterior nasal spine
| Gonial angle [Figure 1d] | Angular measurement between the line from condylion to gonion and the line tangent to lower border of the mandible. | Sagittal |
|---|---|---|
| Interdistance of mandible (LL) [Figure 4a] | Linear distance from the point of one gonion to the point of contralateral gonion. | Axial |
| Sagittal distance of mandible (AP) [Figure 4b] | Vertical line from the anterior most point on the lingual aspect of the mandibular symphysis to a line between both sides of the gonion points | Axial |
| Transverse mandibular angle. [Figure 4c] | Angular measurement between the anterior most point on the mental protuberance and the gonion point on both sides of the mandible. | Axial |
| Tongue volume (mm3) [Figure 5] | Segmentation of the tongue on the ventral aspect: e cervical margin of mandibular posterior teeth will be rotated on the sagittal view in such a manner that the above plane will be parallel to the xaxis plane Segmentation of the tongue on the axial aspect: A right angle plane declining from the PNS in the axial direction will be considered to form the base of the tongue |
PNS: Posterior nasal spine
Statistical analysis
The Statistical software IBM Statistical Package for the Social Sciences 20.0 (IBM Corporation, Armonk, NY, USA) was utilized for data analysis. Analysis of variance, unpaired t-test, and Tukey test were used to evaluate and comparison of facial patterns and skeletal classes. Pearson correlation test was done to determine the correlations between the PSV and the other variables. The level of significance was kept at 0.05.
RESULTS
The intra-examiner intraclass correlation showed values between 0.76 and 0.97 for the angular measurements. The distribution of skeletal Classes is shown in [Table 3]. There were statistically significant differences in all the measured variables (P < 0.001) except for the gonial angle of the mandible, which showed statistically insignificant with P = 0.675.
| Variables | Class I (n=40) | Class II (n=40) | Class III (n=40) | P-value |
|---|---|---|---|---|
| ANS_PNS | 46.250±2.0142 | 47.100±2.0295 | 44.835±1.8556 | <0.001** |
| Constricted distance | 6.485±1.6084 | 5.660±0.9432 | 7.315±1.3975 | <0.001** |
| C1-LL | 24.700±3.5141 | 20.090±3.6382 | 27.355±1.5457 | <0.001** |
| C1-AP | 8.995±3.0739 | 7.275±1.9390 | 10.925±2.1857 | <0.001** |
| C2-LL | 22.655±5.2095 | 19.420±3.5395 | 28.485±2.0807 | <0.001** |
| C2-AP | 7.065±1.9128 | 7.485±1.1360 | 9.940±1.9701 | <0.001** |
| C3-LL | 26.850±3.1011 | 23.740±1.5602 | 30.190±1.4293 | <0.001** |
| C3-AP | 9.130±1.9620 | 7.955±1.5377 | 10.370±1.5881 | <0.001** |
| Epiglottis-LL | 27.300±1.8453 | 24.765±1.2475 | 33.015±3.4038 | <0.001** |
| Epiglottis-AP | 10.195±1.3062 | 8.380±1.4162 | 11.545±1.5536 | <0.001** |
| Airway volume | 8119.6600±561.20097 | 5991.2075±1689.89360 | 11140.0700±1386.48827 | <0.001** |
| Gonial angle | 124.800±8.8729 | 125.650±8.5472 | 126.550±9.0155 | 0.675 |
| Transverse mandibular angle | 62.585±0.7701 | 63.010±0.9703 | 61.660±1.2868 | <0.001** |
| Inter-distance of mandible | 82.375±1.0655 | 83.455±0.3336 | 84.400±0.6854 | <0.001** |
| AP-mandible distance | 57.365±0.7866 | 58.000±0.4961 | 60.620±1.5119 | <0.001** |
| Tongue volume | 42962.235±926.5525 | 39251.695±1996.3261 | 50073.750±1609.6806 | <0.001** |
P<0.001 - Highly significant**. Same alphabets indicate significant difference using Tukey’s post hoc analysis. ANS: Anterior nasal spine, PNS: Posterior nasal spine, ANOVA: Analysis of variance, SD: Standard deviation, CILL: Interspace at C1 lateral to lateral, C1AP: Interspace at C1 anterioposteriorly, C2LL: Interspace at C2 lateral to lateral, C2AP: Interspace at C2 anterioposteriorly, C3LL: Interspace at C3 lateral to lateral, C3AP: interspace at C3 anterioposteriorly.
The distribution of variables in different facial types, which showed highly significant differences (P < 0.001) while tongue volume showed a statistically significant difference at P < 0.05 whereas ANS-PNS, C1-LL distance, C1-AP distance, LL distance of mandible, and AP distance of mandible showed no statistically significant differences (P < 0.05) with P = 0.223, 0.067, 0.123, 0.475, and 0.37, respectively is showed in [Table 4].
| Variables | Hypodivergent (n=60) | Hyperdivergent (n=60) | P-value |
|---|---|---|---|
| ANS_PNS | 46.303±2.1762 | 45.820±2.1450 | 0.223 |
| Constricted distance | 7.323±1.4480 | 5.650±0.9986 | <0.001** |
| C1-LL | 24.763±5.1156 | 23.333±3.0994 | 0.067 |
| C1-AP | 9.467±3.0788 | 8.663±2.5696 | 0.123 |
| C2-LL | 25.317±4.4267 | 21.723±5.6159 | <0.001** |
| C2-AP | 8.780±2.3715 | 7.547±1.6461 | <0.001** |
| C3-LL | 27.933±3.2024 | 25.920±3.3330 | <0.001** |
| C3-AP | 10.053±1.8063 | 8.250±1.6840 | <0.001** |
| Epiglottis-LL | 29.973±4.6182 | 26.747±2.9208 | <0.001** |
| Epiglottis-AP | 10.760±1.9557 | 9.320±1.6094 | <0.001** |
| Airway volume | 9417.8640±2163.30421 | 7416.0943±2392.86501 | <0.001** |
| Gonial angle | 117.433±3.1642 | 133.900±2.6852 | <0.001** |
| Transverse mandibular angle | 61.710±0.9360 | 63.127±0.9264 | <0.001** |
| Inter-distance of mandible | 83.483±0.9603 | 83.337±1.2624 | 0.475 |
| AP-mandible distance | 58.800±1.9162 | 58.523±1.5520 | 0.387 |
| Tongue volume (mm3) | 44969.263±4677.6871 | 43222.523±4742.3647 | 0.044* |
P<0.05 - Significant*, P<0.001 - Highly significant**. ANS: Anterior nasal spine, PNS: Posterior nasal spine, SD: Standard deviation. C1-LL - Interspace at C1 lateral to lateral, C1-AP - Interspace at C1 anterioposteriorly, C2-LL- Interspace at C2 lateral to lateral, C2-AP- Interspace at C2 anterioposteriorly, C3-LL- Interspace at C3 lateral to lateral. C3-AP- Interspace at C3 anterioposteriorly.
As shown in [Table 5], a significant correlation was found between the PSV and the measured variables. In both facial types, the correlation between the parameter’s airway volume and ANS-PNS shows a negative correlation and is statistically significant for P < 0.001 for the hypodivergent pattern (−0.456).
| Airway volume | Total | Class I | Class II | Class III | Hypo divergent | Hyper divergent |
|---|---|---|---|---|---|---|
| ANSPNS | ||||||
| Pearson correlation | −0.289** | −0.080 | 0.338* | 0.103 | −0.456** | −0.287* |
| Sig. (2-tailed) | 0.001 | 0.622 | 0.033 | 0.528 | 0.000 | 0.026 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
| Constricted t distance | ||||||
| Pearson correlation | 0.583** | 0.248 | 0.382* | 0.726** | 0.585** | 0.349** |
| Sig. (2-tailed) | 0.000 | 0.123 | 0.015 | 0.000 | 0.000 | 0.006 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
| C1LL | ||||||
| Pearson correlation | 0.645** | 0.214 | 0.093 | 0.474** | 0.545** | 0.852** |
| Sig. (2-tailed) | 0.000 | 0.185 | 0.569 | 0.002 | 0.000 | 0.000 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
| C1AP | ||||||
| Pearson correlation | 0.526** | 0.188 | 0.381* | 0.039 | 0.405** | 0.650** |
| Sig. (2-tailed) | 0.000 | 0.245 | 0.015 | 0.811 | 0.001 | 0.000 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
| C2LL | ||||||
| Pearson correlation | 0.726** | 0.579** | 0.658** | −0.179 | 0.468** | 0.843** |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.268 | 0.000 | 0.000 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
| C2AP | ||||||
| Pearson correlation | 0.557** | 0.411** | 0.009 | 0.560** | 0.682** | 0.299* |
| Sig. (2tailed) | 0.000 | 0.009 | 0.955 | 0.000 | 0.000 | 0.020 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
| C3LL | ||||||
| Pearson correlation | 0.764** | 0.331* | 0.618** | 0.231 | 0.614** | 0.845** |
| Sig. (2-tailed) | 0.000 | 0.037 | 0.000 | 0.151 | 0.000 | 0.000 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
| C3AP | ||||||
| Pearson correlation | 0.623** | 0.584** | 0.397* | 0.545** | 0.492** | 0.587** |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.011 | 0.000 | 0.000 | 0.000 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
| EpiglottisLL | ||||||
| Pearson correlation | 0.897** | 0.663** | 0.471** | 0.939** | 0.959** | 0.843** |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
| EpiglottisLL | ||||||
| Pearson correlation | 0.740** | 0.449** | 0.407** | 0.556** | 0.643** | 0.764** |
| Sig. (2-tailed) | 0.000 | 0.004 | 0.009 | 0.000 | 0.000 | 0.000 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
| Gonial angle | ||||||
| Pearson correlation | −0.356** | −0.560** | −0.912** | −0.839** | 0.040 | 0.130 |
| Sig. (2-tailed) | 0.000 | 0.000 | 0.000 | 0.000 | 0.762 | 0.322 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
| Transverse angle of mandible | ||||||
| Pearson correlation | −0.696** | −0.409** | −0.771** | −0.605** | −0.706** | −0.542** |
| Sig. (2-tailed) | 0.000 | 0.009 | 0.000 | 0.000 | 0.000 | 0.000 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
| Interdistance of mandible | ||||||
| Pearson correlation | 0.351** | 0.180 | 0.082 | −0.157 | 0.519** | 0.244 |
| Sig. (2-tailed) | 0.000 | 0.266 | 0.614 | 0.333 | 0.000 | 0.060 |
| N | 120 | 40 | 40 | 40 | 60 | 60 |
| AP distance of mandible | ||||||
| Pearson correlation | 0.586** | 0.070 | −0.399* | 0.250 | 0.781** | 0.429** |
| Sig. (2-tailed) | 0.000 | 0.670 | 0.011 | 0.120 | 0.000 | 0.001 |
| N | 120 | 40 | 40 | 40 | 60 | 60 |
| Tongue volume | ||||||
| Pearson correlation | 0.948** | 0.027 | 0.921** | 0.931** | 0.984** | 0.964** |
| Sig. (2-tailed) | 0.000 | 0.869 | 0.000 | 0.000 | 0.000 | 0.000 |
| n | 120 | 40 | 40 | 40 | 60 | 60 |
Positive correlation means as one parameter value increases the other also increases. Negative correlation means as one parameter increases the other decreases. **Correlation is significant at the 0.01 level (2-tailed). *Correlation is significant at the 0.05 level (2-tailed). ANS: Anterior nasal spine, PNS: Posterior nasal spine. C1-LL: Interspace at C1 lateral to lateral, C2-LL: Interspace at C2 Lateral to Lateral, C2-AP: Interspace at C2 anterioposteriorly, C3-LL: Interspace at C3 lateral to lateral, C3-AP: Intersapce at C3 anterioposteriorly
In class III hypodivergent, the correlation between the airway volume and shortest distance (0.726, 0.585, respectively) shows a very good positive correlation and is significant with a P < 0.001. Correlation between the airway volume and transverse angle of the mandible shows excellent negative correlation (−0.409, −0.771, −0.605, −0.706, −0.542, respectively) for class II and class III skeletal classes and both facial types and is statistically significant for P < 0.001.
The correlation between the parameters airway volume and tongue volume (0.921, 0.931, 0.984, and 0.964, respectively) shows an excellent positive correlation and is significant with a P < 0.001 for class II and class III skeletal pattern and in both facial types.
The results of the intergroup comparison of skeletal classes with hypodivergent growth pattern are shown in [Table 6], and all variables showed statistically significant differences (P < 0.001), the following variables showed significantly higher values for the shortest/constricted distance (8.420 ± 0.5307), C1-LL (28.130 ± 0.7935), C1-AP (10.960 ± 1.5581), C2-LL (27.980 ± 1.9509), C2-AP (10.960 ± 2.0423), C3-LL (30.450 ± 1.0566), C3-AP (11.080 ± 1.7392), epiglottis-LL (35.550 ± 3.0050), epiglottis-AP (12.390 ± 1.5), airway volume (12310.140 ± 858.6), gonial angle (118.0 ± 2.15), inter-distance of the mandible (84.320 ± 0.69), and tongue volume (51271.300 ± 1104.7) in Class III in comparison with class I and class II.
| Variables | Class I | Class II | Class III | Intergroup comparison** | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| ANS_PNS | 46.000 | 2.4675 | 47.800 | 1.7205 | 45.110 | 1.3054 | Class II>Class I>Class III |
| Constricted distance | 7.400 | 1.6053 | 6.150 | 0.9649 | 8.420 | 0.5307 | Class III>Class I>Class II |
| C1-LL | 25.900 | 4.5229 | 20.260 | 5.0534 | 28.130 | 0.7935 | Class III>Class I>Class II |
| C1-AP | 9.690 | 3.8850 | 7.750 | 2.5320 | 10.960 | 1.5581 | Class III>Class I>Class II |
| C2-LL | 26.650 | 4.4595 | 21.320 | 3.317 | 27.980 | 1.9509 | Class III>Class I>Class II |
| C2-AP | 8.020 | 1.8266 | 7.360 | 1.4912 | 10.960 | 2.0423 | Class III>Class I>Class II |
| C3-LL | 28.610 | 3.4153 | 24.740 | 1.0870 | 30.450 | 1.0566 | Class III>Class I>Class II |
| C3-AP | 10.330 | 1.3479 | 8.750 | 1.5206 | 11.080 | 1.7392 | Class III>Class I>Class II |
| Epiglottis-LL | 28.930 | 0.6914 | 25.440 | 1.0976 | 35.550 | 3.0050 | Class III>Class I>Class II |
| Epiglottis-AP | 10.810 | 1.2736 | 9.080 | 1.4215 | 12.390 | 1.5697 | Class III>Class I>Class II |
| Airway volume | 8443.9700 | 98.21981 | 7499.4820 | 327.84472 | 12310.1400 | 858.68245 | Class III>Class I>Class II |
| Gonial angle | 116.700 | 4.4260 | 117.600 | 2.4366 | 118.000 | 2.1521 | Class III>Class II>Class I |
| Transverse mandibular angle | 62.030 | 0.4256 | 62.300 | 0.7211 | 60.800 | 0.8208 | Class II>Class I>Class III |
| Inter-distance mandible | 87.680 | 0.9243 | 83.450 | 0.3753 | 84.320 | 0.6971 | Class I>Class III>Class II |
| AP-distance mandible | 57.440 | 0.8586 | 57.820 | 0.6254 | 61.040 | 1.5198 | Class III>Class II>Class I |
| Tongue volume | 42674.700 | 1057.3285 | 40961.790 | 1163.5743 | 51271.300 | 1104.7493 | Class III>Class I>Class II |
(P<0.001 - Highly significant**). ANS: Anterior nasal spine, PNS: Posterior nasal spine, ANOVA: Analysis of variance, SD: Standard deviation, C1-LL: Interspace at C1 lateral to lateral, C1-AP: Interspace at C1 anterioposteriorly, C2-LL: Interspace at C2 lateral to lateral, C2-AP: Interspace at C2 anterioposteriorly, C3-LL: Interspace at C3 lateral to lateral, C3-AP: Interspace at C3 anterioposteriorly.
ANS-PNS (47.8 ± 1.7205) and transverse mandibular angle (62.300 ± 0.7211) showed significantly higher values in Class II compared to Class I and Class III in the hypodivergent growth pattern. Interdistance of the mandible (87.680 ± 0.92) showed significantly higher values for class I compared to class II and class III hypodivergent patterns.
As shown in [Table 7], comparison of skeletal classes with hyperdivergent growth pattern showed statistically higher values for shortest distance, C1-LL, C1-AP, C2-LL, C2-AP, C3-LL, C3-AP, epiglottis-LL, epiglottis-AP, airway volume, and tongue volume in class III compared to class I and class II. ANS-PNS, transverse mandibular angle, and AP mandibular distance showed significantly higher values in class II in comparison with class I and class III. AP-mandible distance showed significantly higher values for class I in comparison with class III and class II.
| Variables | Class I | Class II | Class III | Intergroup comparison** | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| ANS_PNS | 46.400 | 1.4517 | 46.500 | 2.1126 | 44.560 | 2.2814 | Class II>Class I>Class III |
| Constricted distance | 5.570 | 0.9852 | 5.170 | 0.6242 | 6.210 | 1.7355 | Class III>Class I>Class II |
| C1-LL | 23.500 | 1.3634 | 19.920 | 1.2539 | 26.580 | 1.7350 | Class III>Class I>Class II |
| C1-AP | 8.300 | 108122 | 6.800 | 0.9119 | 10.890 | 2.7198 | Class III>Class I>Class II |
| C2-LL | 18.660 | 1.4898 | 17.520 | 2.6667 | 28.990 | 2.1317 | Class III>Class I>Class II |
| C2-AP | 6.110 | 1.5012 | 7.610 | 0.6265 | 8.920 | 1.2672 | Class III>Class II>Class I |
| C3-LL | 25.090 | 1.2468 | 22.740 | 1.3076 | 29.930 | 1.7131 | Class III>Class I>Class II |
| C3-AP | 7.930 | 1.7472 | 7.160 | 1.1004 | 9.660 | 1.0445 | Class III>Class I>Class II |
| Epiglottis-LL | 25.670 | 0.9581 | 24.090 | 1.0151 | 30.480 | 1.1058 | Class III>Class I>Class II |
| Epiglottis-AP | 9.580 | 1.0411 | 7.680 | 1.0319 | 10.700 | 0.9937 | Class III>Class I>Class II |
| Airway volume | 7795.3500 | 644.51288 | 4482.9330 | 982.37185 | 9970.0000 | 571.22730 | Class III>Class I>Class II |
| Gonial angle | 132.900 | 1.9708 | 133.700 | 2.7549 | 135.100 | 2.8819 | Class III>Class II>Class I |
| Transverse mandibular angle | 63.140 | 0.6227 | 63.720 | 0.5926 | 62.520 | 1.0807 | Class II>Class I>Class III |
| Inter-distance Mandible | 82.707 | 1.1314 | 83.460 | 0.3500 | 84.480 | 0.6818 | Class III>Class II>Class I |
| AP-distance Mandible | 87.190 | 0.6843 | 58.180 | 0.2142 | 60.200 | 1.4179 | Class I>Class III>Class II |
| Tongue volume | 43249.770 | 685.6996 | 37541.600 | 818.4130 | 48876.200 | 1038.6684 | Class III>Class I>Class II |
(P<0.001 -Highly significant**). ANS: Anterior nasal spine, PNS: Posterior nasal spine, ANOVA: Analysis of variance, SD: Standard deviation
The simple linear regression equation resulted in the formula
PSV = 67.362 + (23.5 × LLC2) - (14.2 × constricted distance) - (21 × APC3) + (65.4 × inter-distance of mandible) - (38.8 × LLC1) + (20.8 × LL epiglottis) + (35.2 × APC1) + (9.391 × ANS-PNS distance) - (34.164 × LLC3).
The addition of further variables, namely age, sex, skeletal class, or facial pattern, did not refine the equation as shown in [Table 8].
| Variables | Unstandardized coefficients | Standardized Coefficients | t | Sig. | |
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| (Constant) | 67.362 | 5555.303 | 0.012 | 0.990 | |
| ANS_PNS | 9.391 | 27.043 | 0.008 | 0.347 | 0.729 |
| Constricted distance | −14.288 | 52.319 | −0.009 | −0.273 | 0.785 |
| C1-LL | −38.892 | 19.113 | −0.067 | −2.035 | 0.044 |
| C1-AP | 35.269 | 26.046 | 0.041 | 1.354 | 0.179 |
| C2-LL | 23.518 | 15.765 | 0.051 | 1.492 | 0.139 |
| C3-LL | −34.164 | 26.301 | −0.047 | −1.299 | 0.197 |
| C3-AP | −21.003 | 53.789 | −0.017 | −0.390 | 0.697 |
| Epiglottis-LL | 20.898 | 33.177 | 0.035 | 0.630 | 0.530 |
| Inter-distance of mandible | 65.459 | 58.830 | 0.046 | 1.113 | 0.268 |
B: Unstandardized Coefficients, ANS: Anterior nasal spine, PNS: Posterior nasal spine
DISCUSSION
The influence of respiration on the growth of craniofacial structure and its significance in treatment planning has been a controversial topic in the field of orthodontics.[2,7,20] Previous studies have stated that abnormal development of the maxilla-mandibular structures can result in modification of the pharyngeal airway volume, which could lead to changes in soft and hard tissue structures like hyoid bone.[21-23]
Previous study has reported that pharyngeal tissues exhibit growth till the age of 13 years.[10] Most of the longitudinal studies have reported that the muscular palate increases in height and depth between 20 and 50 years. [10,24] Many investigators have utilized lateral cephalometric analysis to evaluate the pharyngeal airway space, identifying the definite hard and soft-tissue landmarks. A major disadvantage is that it lacks accurate description and characteristics of pharyngeal airway space. In this study, we used 3D CBCT imaging technology, which helped us to understand and visualize the 3D airway volume in a better way.[25] Previous studies have reported adaptations in the PSV, such as extended or forwarded head posture, skeletal classes, and facial patterns[26-28], while few authors have assessed the pharyngeal volume in different skeletal classes, although the consequence was questionable.[6,14,29] Kormaz et al.[30] reported no significant difference in cranio-cervical angulation among obese and non-obese subjects without OSA, which might probably influence the head posture, according to body mass index (BMI). From this, we can conclude that there is no significant effect of BMI on the pharyngeal airway.
According to Jayaratne and Zwahlen,[31] there was a significantly larger oropharyngeal volumes in skeletal class III subjects in comparison with skeletal class II subjects, which was in concordance with our research. We consider that these differences among the studies might be due to discrete methodologies, which include variable sample sizes, ethnic groups, examination procedure (2-D or 3D technique), and use of different software programs.
A study by Chianchitlert et al.,[32] reported that subjects with retrognathic mandible exhibited a narrow pharyngeal airway space compared to individuals with normal sagittal skeletal pattern. While small and backwardly rotated mandible probably influences the tongue and the muscular palate in the posterior-inferior direction, which leads to encroach of the muscles of the pharynx in the gonial angle area with a resultant reduction in pharyngeal airway space.[25,33] Above findings were in agreement with our study, as class II hyperdivergent subjects showed a significant negative correlation between PSV and mandibular morphological parameters . Furthermore, a study by Syal et al.[10] stated that the position of the mandible in relationship with the cranial base has an influence on the oropharyngeal area.
Diwakar et al.[34] claimed that there was a direct relationship between the mandibular length and, the oropharyngeal airway and the total pharyngeal airway space. These discovered a relationship between mandibular length and airway size. The present study has exhibited that class III subjects showed higher pharyngeal volume and tongue volume, followed by class I and class II subjects. Since skeletal class III subjects showed more forwardly placed mandible and tongue, thereby increasing the linear dimension between posterior aspects of the tongue and the pharyngeal posterior wall, leading to enlarged pharyngeal volume and tongue volume in class III skeletal malocclusion than compared to skeletal class I and II malocclusion.[35] This was in agreement with studies by Kale and Buyukcavus,[6] Laranjo and Pinho,[36] and Habumugisha et al.[37] They reported that retrognathic class II subjects had reduced nasal pharyngeal and adenoidal tissue.
The current study shows a substantial correlation between the pharyngeal airway capacity and craniofacial characteristics, demonstrating the relationship between form and function. In relation to facial types, a highly significant difference was established in the hyperdivergent growth pattern and hypodivergent growth pattern. Among all the groups and subgroups, the Class III hypodivergent growth pattern showed larger pharyngeal volume and smaller pharyngeal volume was found in the Class II hyperdivergent growth pattern. According to a study done by Opdebeeck et al.,[38] the individuals with a long face have a narrower airway volume due to the reduced cross-section of the hyoid bone and backward positioning of the pharynx nearer to the cervical spine.
Another study by Kale and Buyukcavus[6] stated that subjects with hyperdivergent pattern with Class I and Class II malocclusion had a reduced oropharyngeal airway than subject with normodivergent pattern with Class I and Class II malocclusion. Previous studies have classified variable grades of OSA by assessing linear and angular measurements in the lower jaw, hyoid bone, and pharyngeal space, and they reported that the severity of OSA is more with smaller measurements.[29,39,40] In our research, we measured the pharyngeal space at the area of greatest constriction. The results exhibited that these measurements were lower in Class II hyperdivergent subjects.
Further, to enable the evaluation of PSV by experts without access to or knowledge of segmentation tools, a linear regression equation has been formulated using only linear measurements for the estimation of PVS. The above formula exhibited that our equation is probably helpful for airway volume assessment.
Therefore, we believe that our research has emphasized the significance of the inter-relation and compensation of morphological patterns of the mandible, tongue, and pharyngeal airway volume, and information regarding this will help in orthodontic diagnosis and treatment planning, especially for timely dentofacial orthopedic treatment interventions and need for referral to ENT specialist.
Future research scope
The present study excluded parameters such as body type or BMI to correlate with the growth pattern and pharyngeal airway volume. This research could be a foundational basis where further research should be conducted by considering the respiration phase, the BMI or neck circumference, and body types with respect to pharyngeal airway, tongue volume, and mandibular morphology.
CONCLUSION
Within the scope of the study, it is stated that there is a direct relationship between pharyngeal space, mandibular morphology, and tongue volume.
Among all the subgroups, Class III hypodivergent showed the highest pharyngeal airway volume and tongue volume, and the least was found in Class II hyperdivergent subjects, showing the influence on pharyngeal space, mandibular morphology, and tongue position.
Thus, recommending thorough analysis of oropharyngeal structures in a non-individualized way for orthodontic diagnosis and treatment plan to obtain stable treatment outcomes.
Ethical approval
The research/study was approved by the Institutional Review Board at Al Badar Dental College and Hospital, number No. IEC/2019-20/03, dated 15/05/2019.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Evaluation of pharyngeal space and its correlation with mandible and hyoid bone in patients with different skeletal classes and facial types. Am J Orthod Dentofacial Orthop. 2018;153:825-33.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between the position of hyoid bone and subregions of the pharyngeal airway space in lateral cephalometry and cone beam computed tomography. Angle Orthod. 2017;87:688-95.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of naso-respiratory obstruction with mouth breathing on dentofacial and craniofacial development. Orthod J Nepal. 2018;8:22-7.
- [CrossRef] [Google Scholar]
- Comparative evaluation of the relationship between airway inadequacy, head posture, and craniofacial morphology in mouth-breathing and nasal-breathing patients: A cephalometric observational study. Cureus. 2023;15:e47435.
- [CrossRef] [Google Scholar]
- Influence of resting tongue posture on mandibular arch width and vertical dimensions of the face. J Postgrad Med Inst. 2023;37:195-200.
- [Google Scholar]
- Effect of craniofacial growth pattern on head posture. J Dent Indones. 2020;27:144-50.
- [CrossRef] [Google Scholar]
- Systematic review: Craniocervical posture and craniofacial morphology. Eur J Orthod. 2014;36:55-66.
- [CrossRef] [PubMed] [Google Scholar]
- Soft tissue airway dimensions and craniocervical posture in subjects with different growth patterns. Angle Orthod. 2015;85:604-10.
- [CrossRef] [PubMed] [Google Scholar]
- Airway from an orthodontic perspective. J Clin Diag Res. 2021;15:ZE07-12.
- [CrossRef] [Google Scholar]
- Pharyngeal airway space, hyoid bone position, and head posture after bimaxillaryorthognathic surgery in Class III patients: Long-term evaluation. Angle Orthod. 2014;84:773-81.
- [CrossRef] [PubMed] [Google Scholar]
- The role of cone-beam computed tomography in the radiographic evaluation of obstructive sleep apnea: A review article. Imaging Sci Dent. 2023;53:283-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cone beam computed tomography: Basics and applications in dentistry. J Istanb Univ Fac Dent. 2017;51:S102-21.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship of craniofacial morphology in 3-dimensional analysis of the pharynx. Am J Orthod Dentofacial Orthop. 2016;149:683-91.
- [CrossRef] [PubMed] [Google Scholar]
- CBCT imaging-A boon to orthodontics. Saudi Dent J. 2015;27:12-21.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation and correlation between the pharyngeal space, mandible and tongue in two different facial pattern. J Indian Orthod Soc. 2022;56:351-8.
- [CrossRef] [Google Scholar]
- Determination of vertical characteristics with different cephalometric measurements. Eur J Dent. 2016;10:116-20.
- [CrossRef] [PubMed] [Google Scholar]
- Cone-beam computed tomography evaluation of short-and long-term airway change and stability after orthognathic surgery in patients with Class III skeletal deformities: Bimaxillary surgery and mandibular setback surgery. IntJ Oral Maxillofac Surg. 2012;41:87-93.
- [CrossRef] [PubMed] [Google Scholar]
- Cone-beam computed tomography evaluation of relationship between tongue volume and lower incisor irregularity. Eur J Orthod. 2013;35:555-5.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of craniofacial morphology on the upper airway dimensions. Angle Orthod. 2015;85:874-80.
- [CrossRef] [PubMed] [Google Scholar]
- Computerized cephalometric study of the pharyngeal airway space in patients submitted to orthognathic surgery. J Maxillofac Oral Surg. 2014;13:253-8.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of hyoid bone position and its correlation with pharyngeal airway space in different types of skeletal malocclusion. Contemp Clin Dent. 2014;5:187-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of pharyngeal airway volume in individuals with different skeletal patterns. Meandros Med Dent J. 2021;22:7-17.
- [CrossRef] [Google Scholar]
- A novel approach to determine the prevalence of type of soft palate using digital intraoral impression. Int J Dent. 2017;2017:3268064.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of pharyngeal airway using lateral cephalogram vs CBCT images: A cross-sectional retrospective study. Open Dent J. 2015;9:263-6.
- [CrossRef] [PubMed] [Google Scholar]
- A study on the evaluation of pharyngeal size in different skeletal patterns: A radiographic study. J Contemp Dent Pract. 2018;19:1278-83.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison between growth patterns and pharyngeal widths in different skeletal malocclusions in South Indian population. J Int Soc Prev Community Dent. 2018;8:224-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of pharyngeal airway width among individuals having skeletal class I malocclusion with different growth pattern. Orthod J Nepal. 2022;12:23-7.
- [CrossRef] [Google Scholar]
- Hyoid bone position as an indicator of severe obstructive sleep apnea. BMC Pulm Med. 2022;22:349.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of hyoid bone positions and pharyngeal airway dimensions in different body mass index percentile adolescent subjects. Cranio. 2020;38:286-91.
- [CrossRef] [PubMed] [Google Scholar]
- The oropharyngeal airway in young adults with skeletal class II and class III deformities: A 3D morphometric analysis. PLos One. 2016;11:e0148086.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative assessment of the upper pharyngeal airway dimensions among different anteroposterior skeletal patterns in 7-14-year-old children: A cephalometric study. Children. 2022;9:1163.
- [CrossRef] [PubMed] [Google Scholar]
- Facial morphology and obstructive sleep apnea. Dental Press J Orthod. 2015;20:60-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of craniofacial morphology on pharyngeal airway volume measured using cone-beam computed tomography (CBCT)-A retrospective pilot study. Int J Environ Res Public Health. 2021;18:5040.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of pharyngeal airway volume for different dentofacial skeletal patterns using cone-beam computed tomography. J Dent Sci. 2021;16:51-7.
- [CrossRef] [PubMed] [Google Scholar]
- Cephalometric study of the upper airways and dentoalveolar height in open bite patients. Int Orthod. 2014;12:467-82.
- [CrossRef] [PubMed] [Google Scholar]
- Three-dimensional evaluation of pharyngeal airway and maxillary arch in mouth and nasal breathing children with skeletal Class I and II. BMC Oral Health. 2022;22:320.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative study between the SFS and LFS rotation as a possible morphogenic mechanism. Am J Orthod. 1978;74:509-21.
- [CrossRef] [PubMed] [Google Scholar]
- Upper airway morphology in adults with positional obstructive sleep apnea. Sleep Breath. 2024;28:193-201.
- [CrossRef] [PubMed] [Google Scholar]
- Linear and volumetric airway changes after maxillomandibular advancement for obstructive sleep apnoea. J Oral Maxillofac Surg. 2015;73:1133-42.
- [CrossRef] [PubMed] [Google Scholar]






