Translate this page into:
Genotoxicity and cytotoxicity of lingual bonded retainers coated with silver and titanium dioxide nanoparticles – A randomized controlled trial

*Corresponding author: Sridevi Padmanabhan, Department of Orthodontics and Dentofacial Orthopedics, Sri Ramachandra Dental College, Chennai, Tamil Nadu, India. sridevipadmanabhan@sriramachandra.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Gnansekharan D, Padmanabhan S, Rajan ST, Narsimhan M. Genotoxicity and cytotoxicity of lingual bonded retainers coated with silver and titanium dioxide nanoparticles – A randomized controlled trial. APOS Trends Orthod. doi: 10.25259/APOS_283_2024
Abstract
Objectives
The popularity of fixed retainers is also challenged by the heightened chance of plaque and calculus accumulation which can lead to carious lesions, gingival inflammation, and periodontal disease. Nanotechnology has been used to coat the surface of orthodontic appliances to control the biofilm with promising results although the information on toxicity is limited. This study aimed to evaluate the genotoxicity and cytotoxicity of lingual bonded retainers coated with silver (Ag) and titanium dioxide nanoparticles and compare them with conventional uncoated fixed retainers.
Material and Methods
60 subjects of which 45 patients requiring retainers were randomly allocated to three experimental groups of 15 each (retainers coated with Ag, TiO2 nanoparticles, and uncoated retainers, respectively). A fourth group of 15 patients not orthodontically treated was taken as baseline control. Oral mucosal scrapings from the tongue were taken at three time intervals: At debonding (T0), at 3 months (T1), and at 6 months (T2). The smears were stained with Papanicolaou stain and studied under a light microscope for micronuclei (MN).
Results
All three experimental groups showed an increase in MN counts over a period of 6 months. Group I showed the highest count at T1 and this was significantly greater than Group 3. Group 2 showed the highest count at T2 but this was not significantly different from the other groups.
Conclusion
Lingual bonded retainers coated with Ag and TiO2 nanoparticles are biocompatible and can be used clinically.
Keywords
Genotoxicity
Cytotoxicity
Nanocoating
Lingual bonded retainers
nanocoating
Silver nanocoating
INTRODUCTION
With retention posing an ongoing challenge, fixed retainers have become extremely popular especially since Zachrisson in 1977 proposed the use of multi-stranded wires bonded canine-to-canine retainers.[1] Besides being esthetic and not requiring patient cooperation, there is evidence that using a fixed bonded retainer reduces the chances of lower labial segment relapse.[2]
The downside is an increased chance of plaque and calculus accumulation which can lead to the formation of carious lesions, gingival inflammation, and periodontal disease.[3] Mechanical methods to remove plaque and antimicrobials to control the microflora can be used; however, these methods depend on patient compliance. In recent times, nanoparticles have been explored as coatings on orthodontic appliances or incorporated into bonding materials such as cement and composites. They have proved effective against various microorganisms thereby controlling the formation and composition of the oral biofilm.[4]
TiO2 is a popular nanomaterial due to its high photocatalytic activity resulting in the organic degradation processes and also due to its low cost, chemical stability, and resistance to photo corrosion.[5] Silver (Ag) is a strong disinfectant, having a broad bactericidal spectrum with the ability to cause changes in the bacterial cell membrane leading to cell death.[6]
Although these nanomaterials have antibacterial action on the oral biofilm, the potential cytotoxicity of these materials on normal cells must be considered. Potential hazards are inflammation, necrosis, reactive oxygen species, and apoptosis. Nanoparticles used in the oral cavity can be absorbed and their small size allows them to be transported to other sites in the body.[7] The increased cytotoxicity of smaller particles is attributed to the “Trojan horse effect.”[7]
A study on human periodontal fibroblasts has proved that Ag nanoparticles of size <20 nm are more cytotoxic than the size of 80–100 nm.[8] Similarly, it has been demonstrated that the cytotoxicity of TiO2 nanoparticles also differs with size, with 5 nm proving more cytotoxic than 32 nm.[9]
The study of cytotoxicity of TiO2, Ag, and other nanoparticles has been restricted to in vitro and cell line studies.[10-13]
Genotoxicity is the ability of an agent to exert adverse effects on the cell’s genetic material whereas cytotoxicity is the ability of an agent to be virulent to living cells.[14] The micronuclei
(MN) index is considered one of the standard cytogenetic endpoints and biomarkers in genetic toxicology. [15,16]
MN are extranuclear cytoplasmic bodies that have their origin from the acentric chromosome fragments, acentric chromatid fragments, or whole chromosomes that fail to be included in the daughter nuclei at the completion of telophase during the process of mitosis because they failed to attach properly with the spindle during segregation process in anaphase.[17]
Objectives
Thus, this study was conceived to evaluate the genotoxicity and cytotoxicity of multistranded lingual bonded retainers coated with Ag and TiO2 nanoparticles in an in vivo environment and compare them with conventional uncoated fixed retainers using the MN assay.
MATERIAL AND METHODS
Trial design
This was a randomized parallel study design with an allocation ratio of 1:1.
Participants
The study was conducted in the Department of Orthodontics, Faculty of Dental Sciences, Sri Ramachandra University, Chennai with approval from the Institutional Ethics Board: CSP/16/SEP/51/281.
Patients with permanent dentition who had completed fixed orthodontic treatment with all teeth present in the lower anterior segment and clinically healthy oral mucosa were included in the study. The age of the patients ranged from 14 to 25 years. Patients who had a history of smoking, oral or systemic diseases, or under any medications or supplements were excluded from the study.
Enrollment
The sample size was calculated using the alpha significance level of 0.05 with a power of 80 as per the study of Natarajan M et al.[15] A total number of 60 subjects were enrolled in the study. Among them, 45 patients who had completed fixed orthodontic treatment and required retainers were recruited into three groups that received intervention. A fourth group of 15 patients (within the same age group) who were not orthodontically treated made up the baseline control [Figure 1].
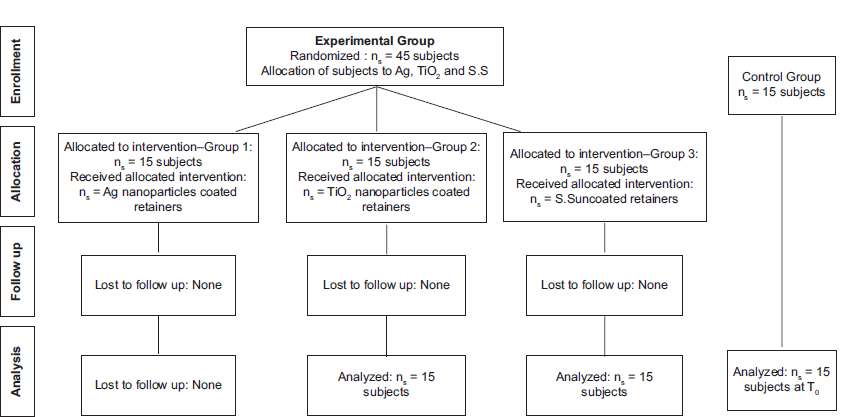
- Consort flow chart. Ag: Silver, TiO2: Titanium dioxide, S.S: Stainless steel
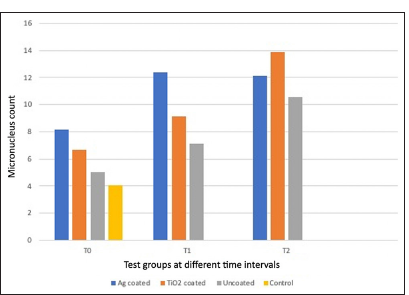
- Comparison of micronuclei count between groups at different time intervals.
Randomization
Randomization was done using the computer-generated program, i.e., www.random.com and an allocation sequence was generated to distribute the intervention methods equally. Patients in the experimental groups were randomly divided into 3 groups of 15 patients each [Table 1].
| Groups | Allocated Intervention |
|---|---|
| Group 1 | Lingual bonded retainer coated with Ag nanoparticles |
| Group 2 | Lingual bonded retainer coated with TiO2 nanoparticles |
| Group 3 | Uncoated stainless steel (S.S) lingual bonded retainer |
| Group 4 | Untreated controls at T0 |
Ag: Silver, TiO2: Titanium dioxide
Blinding
The participants were blinded throughout the study period.
Sample preparation
45 wires of 10 cm length of commercially available 0.017-inch diameter coaxial multi-stranded wire (Rabbit Force Multistranded Retainer, Libral Traders Pvt., Ltd.) were used to prepare the fixed retainers. Of these, 15 samples were coated with Ag nanoparticles, 15 samples were coated with nitrogen (N)-doped TiO2 nanoparticles, and 15 samples were uncoated.
Preparation of retainer wires coated with N-doped TiO2
Surface coating of coaxial multi-stranded retainer wires with N-Doped TiO2 was carried out by the Radio Frequency (RF) magnetron sputtering (Anelva Sputtering Unit Model SPF-332H) method. The wires were coated with TiO2-N of 32 nm size and 99.99% purity and then cooled to room temperature and annealed in a Nitrogen atmosphere at 450°C in a muffle furnace (Sastha Scientific, Bangalore). After annealing, the coated wires were analyzed under a Scanning Electron Microscope, and a film thickness of 50–80 nm TiO2 was observed. X-ray diffractionometer analysis was done to ensure that TiO2 existed in the anatase phase. The wires were then activated in a chamber by visible light (100 W) for 24 h before placing them into the oral cavity.[10]
Surface coating of coaxial multistranded retainer wire with Ag nanoparticles was carried out similarly. The wires were coated with a particle size of 80–100 nm, 99.99% purity, and thickness in the range of 60–80 nm of Ag.
Bonding of fixed retainers
After oral prophylaxis, etching of enamel was done with 37% phosphoric acid gel (d-tech® gel), followed by rinsing with water and drying. Primer (Meta P& Bond) was applied and cured using a Quartz Tungsten Halogen light unit (QLH75 Dentsply) for 10 s/tooth. The retainers were bonded from canine to canine using conventional orthodontic flowable adhesive (Meta Biomed). The adhesive was light cured for 40 s/tooth.
Oral mucosal cell sampling
The cells were collected from each individual using a sterile cement spatula. The blunt end of the spatula was used to perform the motion 2–3 times with a firm force until sufficient material was collected on the edges of the spatula. The end of the spatula was placed on the glass slide and smeared in a single sweep unidirectionally to obtain a perfect smear without clubbing or folding of squamous cells. For the experimental groups, the samples were collected in 3 time periods.
T0-Immediately after debonding of fixed orthodontic appliances subsequent to oral prophylaxis.
T1-3 months after retainer placement T2-6 months after retainer placement.
Samples were collected from untreated participants and compared to the T0 samples.
Slide preparation
The sample obtained was smeared onto the center of a clean glass slide and the smears were immediately fixed in absolute alcohol (Isopropyl alcohol - 100%) for a period of 20–30 min. Then, the slides were hydrated with distilled water and stained using the Papanicolaou (PAP) method according to the standard protocol (PAP, 1942).[18]
The staining technique results in the blue-black appearance of nuclei and the blue-green appearance of cytoplasm. The keratinizing cells have a pinkish-orange hue.
The slides were observed at 40× magnification under the light microscope (Lawrence and Mayo XSZ-N107T). Cells were observed using high-power magnification in 10 fields in a zigzag fashion to determine the presence of MN [Figure 3].
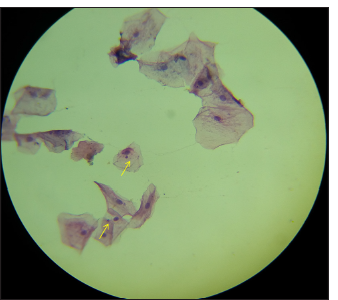
- Micronuclei stained with papanicolaou (PAP) highlighted in high-power magnified field.
Evaluation of MN
MN was identified according to the standard protocol[19] and to fulfill the following characteristics:
Round, smooth perimeter suggesting a membrane
Less than a third of the diameter of the associated nucleus, but large enough to discern the shape and color
Staining intensity similar to that of the nucleus
The same focal plane as the nucleus
No overlap with, or bridge to, the nucleus.
No overlap of the cells.[19]
The number of MN in all the groups was determined and subjected to statistical analysis.
Statistical analysis
The collected data were analyzed with IBM, the Statistical Package for the Social Sciences statistics software 23.0 Version. To describe the data descriptive statistics, mean and standard deviation were used. To find the significant difference within the groups, at different time intervals, the Friedman test was used followed by the Wilcoxon signed-rank test. To find the significant difference between the groups, the Kruskal–Wallis test followed by the Mann–Whitney U-test was used for the intergroup comparison (P < 0.05).
RESULTS
Outcomes
Intragroup comparisons
In group 1, the MN count increased from T0 to T1 and decreased from T1 to T2. The overall increase from T0 to T2 was not statistically significant.
In group 2, the MN count increased sequentially from T0 to T1 to T2. The increase was statistically significant from T0 to T2 and from T1 to T2.
In group 3, the MN count increased sequentially from T0 to T1 to T2. The increase was statistically significant overall from T0 to T2 and T1 to T2 [Tables 2 and 3].
| Time period | Groups | n | Mean | Standard deviation | Mean ranks | Chi-square | P -value |
|---|---|---|---|---|---|---|---|
| T0 | Ag | 15 | 8.13 | 6.896 | 33.77 | 3.360 | 0.339 |
| TiO2 | 15 | 6.67 | 5.052 | 34.57 | |||
| S.S | 15 | 5.00 | 3.162 | 29.43 | |||
| Control | 15 | 4.07 | 1.751 | 24.23 | |||
| T1 | Ag | 15 | 12.40 | 5.742 | 30.53 | 9.521 | 0.009* |
| TiO2 | 15 | 9.13 | 3.204 | 22.67 | |||
| S.S | 15 | 7.13 | 3.248 | 15.80 | |||
| T2 | Ag | 15 | 12.13 | 4.719 | 23.47 | 2.893 | 0.235 |
| TiO2 | 15 | 13.87 | 5.927 | 26.80 | |||
| S.S | 15 | 10.53 | 1.922 | 18.73 |
S.S: Stainless steel, Ag: Silver, TiO2: Titanium dioxide
| Groups | Friedman test | Wilcoxon signed-rank test | ||
|---|---|---|---|---|
| T0-T2 | T0 versus T1 | T1 versus T2 | T0 versus T2 | |
| Group 1 | 0.169 | 0.102 | 0.155 | 0.925 |
| Group 2 | 0.002* | 0.083 | 0.006* | 0.003* |
| Group 3 | 0.001* | 0.131 | 0.001* | 0.009* |
Intergroup comparison
At both T0 and T1, the MN count was highest in group 1 followed by group 2 and group 3. All experimental groups showed a greater MN count as compared to the baseline control at T0 but this was not significant [Figure 2]. At T1, there was a significant difference between group 1 and group 3. At T2, the MN count was greatest in Group 2 followed by Group 1 and Group 3 but this was not statistically significant [Table 4].
| Time period | Ag and TiO2 | Ag and S.S | TiO2 and S.S |
|---|---|---|---|
| T0 | 0.870 | 0.412 | 0.436 |
| T1 | 0.056 | 0.004* | 0.089 |
| T2 | 0.512 | 0.345 | 0.089 |
S.S: Stainless steel, Ag: Silver, TiO2: Titanium dioxide
Harms
The participants in the study did not present with any allergic or adverse reactions during the course of the study.
DISCUSSION
Fixed retention brings associated challenges of maintaining oral hygiene and increased plaque and microbial accumulation on the tooth surface.[4]
The application of nanocoatings on orthodontic appliances has been widely explored and found effective but most studies have been restricted to in vitro situations without evaluating the toxicity. This study sought to evaluate the genotoxicity and cytotoxicity of multistranded lingual bonded retainers coated with Ag and TiO2 nanoparticles in an in vivo environment.
The photocatalytic activity of TiO2 when exposed to ultraviolet (UV) light has been employed since TiO2 has a wide bandgap of 3.2 eV, where its absorption edge occurs below 400 nm (UV region) and only a small fraction of solar spectrum is absorbed.[20] However, since exposure to UV light has its downsides, doping using non-metal ion elements has been favored to reduce the optical gap of TiO2 to visible light. Nitrogen has gained popularity and it has been suggested by Asahi et al.[21] that visible light of <500 nm would be sufficient to activate N-doped TiO2. Since its efficacy has been proven in clinical settings, we chose N-doping to activate TiO2 under visible light.[20] Since the anatase phase has more photocatalytic activity and minimal cytotoxic effects as compared to the rutile phase, we chose to coat our wires with the anatase phase of TiO2.[10]
Ag nanoparticles have a broad bactericidal spectrum and have been used to coat orthodontic appliances including fixed and removable retainers demonstrating strong antibacterial effects.[22,23]
In our study, the coating of the retainer wires was done using a magnetron sputtering machine since this technique has certain advantages such as a strong and uniform coating and high hydrophilicity.[24] While previous studies have used buccal mucosal cells to evaluate genotoxicity, in this study, samples were collected from the tip of the tongue due to its direct contact with the lingual surface of the bonded retainer. MN assay is considered a simple, sensitive, and noninvasive method for evaluating DNA damage, proliferation of basal cells, and cell death. The presence of MN is indicative of chromosomal abnormalities that include breakage of chromosomes and subsequent damage to the DNA. This test is generally performed as a reliable screening test for the presence of genotoxic compounds.[25]
A recent study concluded that PAP stain is the preferred method for detecting MN and this method was used. It encompasses a fixative that has the potential to demarcate the cell boundaries clearly so that the MN is visible in the transparent cytoplasm.[26]
Since orthodontic appliances can also contribute to genotoxicity, we used an untreated control to mitigate the residual influence of fixed appliances for baseline comparison.[14] At T0, all three experimental groups and the untreated control group showed some presence of MN. Although the latter displayed the least MN count compared to the experimental group, the difference was not statistically significant.
At T1, all three experimental groups showed an increase in the MN count, with group 1 (Ag) coated retainers showing the highest count and this was significant when compared to the uncoated retainers.
At T2, there was a further increase in all the groups except group 1, which showed a small and insignificant decrease. There was no significant difference between the three groups.
It appears that Ag nanoparticles were more cytotoxic in the initial periods which reduced over the 3–6-month period. Although TiO2-coated retainers showed a significant increase in the MN over 6 months, this was not significant when compared to the other groups since the uncoated samples group also showed an increase in the number of MN. This could probably be attributed to the inherent cytotoxicity of the metal and mechanical irritation caused by the retainer in contact with the tongue.[14] Although both Ag and TiO2 nanoparticles showed a significant increase over a period, the result was no different when compared to the uncoated stainless steel retainer group.
Despite statistical significance reflected at some time intervals, the increase in MN count from T0 to T2 was not high and leveled off by 6 months. The lack of clinically significant genotoxicity and cytotoxicity when compared with the uncoated retainer group advocates the usage of Ag and TiO2 nanoparticles in orthodontic practice.
Limitations
This study evaluated the cytotoxicity based on the premise that nanocoatings remain viable on the surface of the retainers over 6 months which was not confirmed. The presence of fixed appliances in the mouth can contribute to genotoxicity which is generally reversed. This was mitigated by including an untreated sample.
Generalizability
This is the first study that has reported on the cytotoxicity and genotoxicity of retainers coated with Ag and TiO2 nanoparticles over a period of 6 months. Although the coated retainers did show an increased MN response as compared to non-coated retainers, this was not remarkably significant both statistically and clinically.
CONCLUSION
This study was done to evaluate and compare the genotoxicity and cytotoxicity of lingual bonded retainers coated with Ag and TiO2 nanoparticles. The following conclusions were drawn from the study.
Ag and TiO2 nanoparticles coated retainers showed a significant increase in MN count over a period of 6 months although this increase was no different when compared to uncoated stainless-steel retainers.
Thus, orthodontic lingual bonded retainers coated with Ag and TiO2 nanoparticles are biocompatible and can be used clinically.
Ethical approval
The research/study was approved by the Institutional Review Board at Sri Ramachandra Institute of Higher Education and Research, number CSP/16/SEP/51/281, dated September 2016.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Clinical experience with direct-bonded orthodontic retainers. Am J Orthod. 1977;71:440-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of lingual retainers bonded to the canines in preventing mandibular incisor relapse. Am J Orthod Dentofacial Orthop. 2008;134:179-e1-8.
- [CrossRef] [Google Scholar]
- The association of orthodontic treatment and fixed retainers with gingival health. J Periodontol. 2008;79:2087-92.
- [CrossRef] [PubMed] [Google Scholar]
- Nanoparticles in orthodontics, a review of antimicrobial and anti-caries applications. Acta Odontol Scand. 2014;72:413-7.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95-101.
- [CrossRef] [PubMed] [Google Scholar]
- Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol. 2014;11:11.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro cytotoxicity of silver nanoparticles on human periodontal fibroblasts. J Clin Pediatr Dent. 2011;36:37-41.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of the cytotoxicity of differentially sized titanium dioxide nanoparticles in murine MC3T3-E1 preosteoblasts. J Mater Sci Mater Med. 2011;22:1933-45.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of antibacterial and cytotoxic effects of orthodontic stainless steel brackets coated with different phases of titanium oxide: An in-vitro study. Am J Orthod Dentofacial Orthop. 2017;151:678-84.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro cytotoxicity assessment of an orthodontic composite containing titanium-dioxide nano-particles. J Dent Res Dent Clin Dent Prospects. 2013;7:192-8.
- [Google Scholar]
- Evaluation of the coating with TiO2 nanoparticles as an option for the improvement of the characteristics of NiTi archwires: Histopathological, cytotoxic, and genotoxic evidence. J Nanomater. 2018;2018:2585918.
- [CrossRef] [Google Scholar]
- Assessment of shear bond strength and cytotoxicity of orthodontic adhesive with the addition of silver nanoparticles in varying concentrations. Nanosci Nanotechnol Asia. 2022;12:62-71.
- [CrossRef] [Google Scholar]
- Evaluation of the genotoxic effects of fixed appliances on oral mucosal cells and the relationship to nickel and chromium concentrations: An in-vivo study. Am J Orthod Dentofacial Orthop. 2011;140:383-8.
- [CrossRef] [PubMed] [Google Scholar]
- The micronucleus assay in human buccal cells as a tool for biomonitoring DNA damage: The HUMN project perspective on current status and knowledge gaps. Mutat Res. 2008;659:93-108.
- [CrossRef] [PubMed] [Google Scholar]
- Cytotoxic and genotoxic effects of resin monomers in human salivary gland tissue and lymphocytes as assessed by the single cell microgel electrophoresis (Comet) assay. Biomaterials. 2006;27:1762-70.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the genotoxicity and cytotoxicity in the buccal epithelial cells of patients undergoing orthodontic treatment with three light-cured bonding composites by using micronucleus testing. Korean J Orthod. 2014;44:128-35.
- [CrossRef] [PubMed] [Google Scholar]
- Bancroft's theory and practice of histological techniques (8th ed). Netherlands: Elsevier; 2019. p. :132.
- [Google Scholar]
- Micronuclei and other nuclear anomalies in buccal smears: Methods development. Mutat Res. 1992;271:69-77.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of nitrogen-doped titanium dioxide-modified stainless steel brackets on Streptococcus mutans: A randomized clinical trial. Angle Orthod. 2022;92:396-401.
- [CrossRef] [PubMed] [Google Scholar]
- Visible-light photocatalysis in nitrogen-doped titanium oxides. Science. 2001;293:269-71.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of silver ion coating of fixed orthodontic retainers on the growth of oral pathogenic bacteria. Dent Mater J. 2014;33:268-74.
- [CrossRef] [PubMed] [Google Scholar]
- Streptococcus mutans counts in patients wearing removable retainers with silver nanoparticles vs those wearing conventional retainers: A randomized clinical trial. Am J Orthod Dentofacial Orthop. 2016;149:155-60.
- [CrossRef] [PubMed] [Google Scholar]
- Preparation of an orthodontic bracket coated with an nitrogen-doped TiO2-xNy thin film and examination of its antimicrobial performance. Dent Mater J. 2013;32:311-6.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of Papanicolaou stain for studying micronuclei in buccal cells under field conditions. Acta Cytol. 2006;50:398-402.
- [CrossRef] [PubMed] [Google Scholar]
- Micronuclei assay of exfoliated oral mucosal cells: A review. Ann Dent Special. 2014;2:2.
- [Google Scholar]







