Translate this page into:
Simple method of testing polymer leaching from thermoplastic sheets used for clear aligner
Address for correspondence: Dr. Shuvana Altaf Ansari, B/305, Kinara Apartments, Yari Road, Andheri West, Mumbai - 400 061, Maharashtra, India. E-mail: shuvanaansari@gmail.com.
This article was originally published by Wolters Kluwer and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
With the increasing popularity of clear aligners, newer materials are introduced in the form of thermoplastic sheets. Long-term studies testing the biocompatibility of these sheets are not available. The purpose of this study is to provide a simple, yet effective method to evaluate their leaching potential.
Materials and Methods
Using the oxidation reduction reaction taking place in the presence of two different concentrations of potassium permanganate, five different samples were tested. The sequence of colour change from purple to red to brown to yellow to clear was used to determine the amount of leaching. This is the result of a stoichiometric relation between the oxidizing agent and the reducing sample. Color changes were observed through naked eyes over a period of 24 h. Photographs were taken at 15 min, 8 h and 24 h using D-SLR1000 Canon camera. Photos were evaluated separately.
Result
Diluted solution showed faster change in color, due to the lesser amount of reagent. 3A Medes (Korea) was the first to leach, followed by CA (Scheu, Germany) EVA (Endent Private Limited, Delhi, India), Ultradent (United States) and Avac R (Jaypee, Kerela, India).
Conclusion
“Do it yourself” test is a repeatable and reproducible method that can be modified and used in clinical practice as a chair side method.
Keywords
Do it yourself test
leaching
thermoplastic sheets
INTRODUCTION
In 1980s, vacuum formed clear thermoplastic sheets fitting tightly over the teeth were introduced into orthodontics as “retainers”. It quickly became apparent that if the teeth were slightly reset before forming, a tooth moving device would result; hence the name “aligner” was used. Soon it was apparent that it could be used for far more than just minor movements.[1] Today it is on the verge of bringing about a paradigm shift from unaesthetic brackets and archwires to invisible orthodontic treatment.
Along with the increase in its usage, the duration of oral tissue contact of these plastic sheets have increased, thus proportionately increasing concern on its biocompatible properties. Not much research data is available on this topic. The methods used in previous studies like gas chromatography, mass spectrometry and high performance liquid chromatography are very elaborate, expensive, require specific machinery and trained personnel, which are impractical in clinical practice.[2,3] Plus a wide variety of thermoplastic sheets are available in the market to choose from, with newer brands and materials emerging everyday. The companies’ confidentiality policies make the exact content unknown to the users, thus raising questions. Hence, there is a need for a simpler and inexpensive chair side method which can be used to find the least leaching product out of the options available.
MATERIALS AND METHODS
In this study, we used what we call the ‘do it yourself test’ using potassium permanganate (KMnO4) reagent.[4-6] This is a simple test which can be performed by the clinician or an assistant in the clinic itself using easily available materials. We performed this test in 2 parts differing only in the concentration on the reagent used. In the first part, the reagent was made by adding 1.6 g potassium.
KMnO4 American Chemical Society reagent grade, 99% (0.001 m solution) to 1 L of distilled water, was added. After stirring, the solution was left for at least an hour before use. In the second part of the study the reagent was diluted 1:3 with distilled water.
For sample collection; 20 different dental laboratories using thermoplastic sheets were consulted. Five most commonly used brands were shortlisted for this study. Materials tested: CA, Scheu, Germany; Ultradent, United States; 3A Medes, Korea; Avac R, Jaypee, Kerala; EVA, Endent Pvt. Ltd., Delhi. All samples selected were 2 mm in thickness of medium variety. The samples were 2 cm by 4 cm pieces which were cut out from the excess material left after manufacturing the aligners, hence they had undergone the usual heating and cooling cycle each sheet undergoes before delivery.
All sheets were powdered using straight handpiece with stainless steel round bur (Dentsply, United States) 1 mm in diameter. 1 g of each powdered sheet was added to 5 ml of the reagent in capped and marked test tubes. Color changes were observed through naked eyes over a period of 24 h at 15 min, 8 h and 24 h interval. The test was repeated 5 times for concentrated as well as diluted reagents, over a period of 10 days. Photographs were taken at 15 min, 8 h and 24 h using D-SLR1000 Canon (Canon India private limited, Gurgaon, India) camera. Photos were evaluated separately.
RESULT
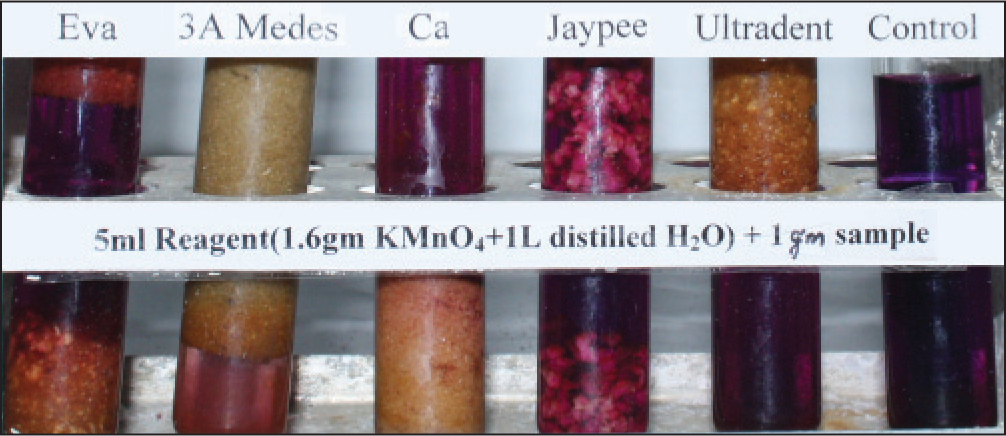
All the products tested leached, but the amount varied from one product to another [Figures 1-6]. The results were consistent on repeated testing over 10 days. Within 15 min, 3A Medes was the first to start changing color in the concentrated solution and had already completed the reaction and turned colorless in the diluted solution. It was followed by CA, next was EVA, Ultradent, Jaypee. The color changes were consistent after 8 and 24 h. The diluted reagent showed faster results than concentrated.
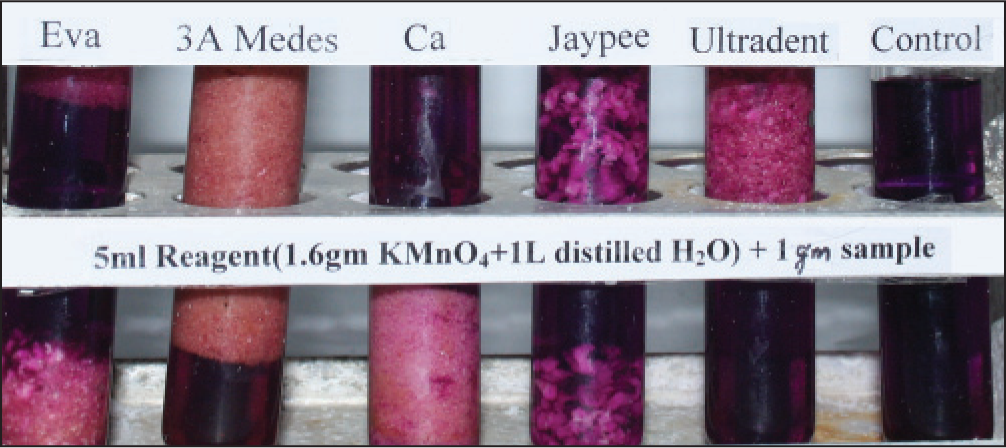
- Sample in reagent after 15 min

- Sample in reagent after 8 h
- Sample in reagent after 24 h

- Sample in diluted reagent after 15 min
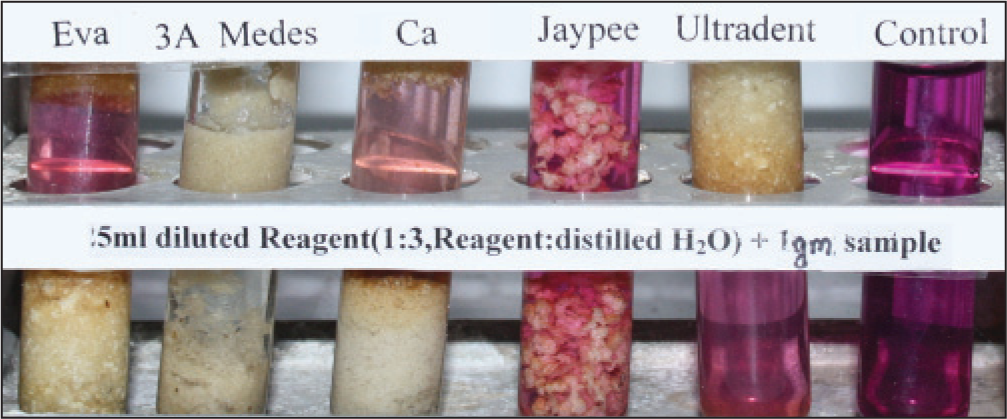
- Sample in diluted reagent after 8 h

- Sample in diluted reagent after 24 h
DISCUSSION
In this study, a simple do it yourself test was used in which an oxidation reduction reaction using KMnO4 takes place.[4] In a neutral environment, the permanganate in the presence of polymers is reduced to manganese (IV) oxide (MnO2) and manganese (III) oxide (Mn2O3) which precipitate and give the liquid a yellow-brown color, manganese is further transformed into a nearly colorless salt (Mn2+). The amount of polymer in the solution determines the degree of progress of reaction; the sequence of color is from purple to red to brown to yellow to clear. This is the result of a stoichiometric relation between the oxidizing agent and the reducing sample.
In this study, we made two readings differing in concentration of reagent in order to check if we could increase the rate of the reaction for clinical usage and still get consistent results. Diluted solution did show faster results, because it had lesser concentration of KMnO4, hence it requires lesser amount of leaching products to complete the reaction and hence shows faster changes in color. The results were similar to the ones obtained using concentrated reagent but occurred at a faster rate.
Based on the facts above, a chair side do-it-yourself test can be put together using potassium KMnO4 which is a common disinfectant that is easily available. Instead of weighing, a solution of permanganate of appropriate concentration can be made by adding water to the potassium KMnO4 crystals until a newspaper could be read through an inch of solution held in a glass tube. A tablespoon of this solution can be used with a pinch of powder for general clinical use.
We found in this study that polymer leaching was seen in all products; however, the rate of polymer leaching differed greatly. This study only provided an approximation of the degree to which a polymer leached oxidizable, organic matter. Although the latter may not always be harmful, as a general rule the less leaching, the safer the product is considered. By showing the relative degree of leaching, the method allows the manufacturer to optimize his product, the clinician and the researcher to select the least harmful and the researcher to follow the progress of the leaching in time.
The variable degree of leaching seen could be due to various reasons. According to Matasa, as the molecular weight of the polymer is increased and the crosslinked network gets denser the larger molecules become entangled and are kept within the polymer’s structure thus reduced amount of leaching.[7]
Thermoplastic overlay orthodontic appliances are generally recommended to be used for 2 weeks in each stage. During this period, temperature fluctuations in the oral cavity can change the properties of these materials.[2] It was reported by Waked et al.[8] that aging induced various dimensional changes in mouthguard materials, which have similar compositions to those of thermoplastic overlay orthodontic materials, depending on the materials and processing techniques.
In contrast to our study, in a study by Schuster et al., in vitro aged and retrieved Invisalign appliances were found to leach no traceable amount of substances in an ethanol aging solution, this was basically attributed to composition of Invisalign appliances, which was found to be composed of polyurethane with added methylene diphenyl diisocyanate and 1, 6 hexanediol.[2] The diphenyl structure provides stability and sufficient reactivity to form a polymer free of byproducts. However it was also added that it did not reflect its in vivo performance, due to various factors like potential abrasion from chewing action, along with attrition induced by the consumption of acidic beverages and the action of enzymes.
In dentistry, bisphenol A (BPA) is a basic material for dental resins that is a precursor for BPA glycidyl dimethacrylate and BPA dimethacrylate. The reverse process of degradation is accelerated with heat, mechanical wear and bacterial or salivary enzymatic action.[9]
Leaching from thermoplastic sheets generally results in the release of monomers such as BPA. The implications of BPA released from dental biomaterials were first reported in a study that assessed dental sealants. BPA is known to cause skin allergies, adverse effects on the reproductive systems of animals, cell death via necrosis and high hemolytic activity.[10-13] In their study Terhune et al. have suggested that clinicians should extend caution in preventing extended contact of any of these materials with a patient’s skin, mucosa and gingiva. BPA is also a well-known potent endocrine disruptor with a weak estrogenic effect.[14]
In a study done by Schuster et al., no cytotoxic or estrogenic activity of Invisalign appliances was documented in in vitro assay, which used a standard model for the assessment of estrogenicity of materials.[2]
Other thermoplastic overlay appliances including retainers, night guards, temporomandibular joint splints and bleaching trays are used for months in the oral cavity, which makes their biocompatibility of utmost importance.[15-19] Kwon et al. reported that there is change in force and energy delivery properties of thermoplastic material after thermocycling or load cycling after three-point bending-recovery test was performed, which shows that by increasing the duration of use, the degradation of polymer could occur at different, possibly faster rate.[15]
The shortcoming of this method is that it gives only comparative results based only on visual estimates; the exact amount of polymer leaching cannot be evaluated. There is a need for a much more accurate but simple method for evaluation of polymer leaching from clear aligner sheets, especially considering its increasing demand.
CONCLUSION
The color fading of the permanganate solutions is a time-proven method to measure the purity of many chemical substances and allows discriminating dental plastics using simple means. Do it yourself test is a simple and effective method that could be used routinely to screen and select most biocompatible clear aligner and other thermoplastic sheets.
Source of Support:
Nil.
Conflict of Interest:
None declared.
References
- Contemporary orthodontic appliances In: Proffit WR, Fields HW, Sarver DM, eds. Contemporary Orthodontics (4th ed). St. Louis, MO: Mosby Elsevier; 2007. p. :402-4.
- [Google Scholar]
- Structural conformation and leaching from in vitro aged and retrieved Invisalign appliances. Am J Orthod Dentofacial Orthop. 2004;126:725-8.
- [Google Scholar]
- Bisphenol A release from an orthodontic adhesive and its correlation with the degree of conversion on varying light-curing tip distances. Am J Orthod Dentofacial Orthop. 2011;140:239-44.
- [Google Scholar]
- Effects of aging on the dimensional stability of custom-made mouthguards. Quintessence Int. 2002;33:700-5.
- [Google Scholar]
- Release of bisphenol A from resin composite used to bond orthodontic lingual retainers. Am J Orthod Dentofacial Orthop. 2011;140:779-89.
- [Google Scholar]
- Occupational health problems and adverse patient reactions in orthodontics. Eur J Orthod. 1989;11:254-64.
- [Google Scholar]
- Leached components from dental composites and their effects on fertility of female mice. Eur J Oral Sci. 2004;112:267-72.
- [Google Scholar]
- Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts. Arch Toxicol. 2006;80:370-7.
- [Google Scholar]
- Studies on hemolytic activity of bisphenol A diglycidyl methacrylate (BIS-GMA) J Dent Res. 1978;57:98-102.
- [Google Scholar]
- In vitro cytotoxicity of orthodontic bonding materials. Am J Orthod. 1983;83:501-6.
- [Google Scholar]
- Force delivery properties of thermoplastic orthodontic materials. Am J Orthod Dentofacial Orthop. 2008;133:228-34.
- [Google Scholar]
- Essix retainers: Fabrication and supervision for permanent retention. J Clin Orthod. 1993;27:37-45.
- [Google Scholar]






