Translate this page into:
The effect of platelet-rich plasma on midpalatal suture in rapid maxillary expansion: An experimental study

*Corresponding author: Hasan İlhan Mutaf, Department of Orthodontics, Cafediş Oral and Dental Health Polyclinic, Kayseri, Turkey. doktormutaf@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Mutaf HI, Biçakçi AA, Gümüş C, İnan VS. The effect of platelet-rich plasma on midpalatal suture in rapid maxillary expansion: An experimental study. APOS Trends Orthod. doi: 10.25259/APOS_320_2024
Abstract
Objectives
Our study aims to examine, histologically and radiologically, the effects of platelet-rich plasma (PRP) applied locally into the midpalatal suture on bone formation during and after rapid maxillary expansion (RME) in rabbits.
Material and Methods
A total of 24 healthy male New Zealand rabbits were randomly divided into three groups. All animals were subjected to 7-day maxillary expansion and 14-day retention. In the control group, physiological saline was injected into the midpalatal suture on the 1st day of the retention. Group I received a PRP injection only 1 time just before retention. In group II, the PRP injection was given twice, once 24 h after the start of the expansion and once on the 1st day of the retention. Radiographs were taken before and after the expansion and retention to assess the suture’s new bone density. All subjects in three groups were evaluated and compared histologically at the end of retention.
Results
A decrease in bone density after expansion and an increase in bone density after retention were seen in all groups. The decrease in bone density after expansion was the least in Group II, and the highest increase in bone density after retention was again in Group II. Inflammation, capillary dilatation, cell infiltration, osteoblastic activity, osteoclastic activity, bone morphogenetic protein-4, and transforming growth factor-beta scores of Group II were significantly higher than the control group.
Conclusion
Locally applied PRP during and after RME may contribute to bone formation in the midpalatal suture.
Keywords
Bone density
Bone morphogenetic protein-4
Platelet-rich plasma
Rapid maxillary expansion
Transforming growth factor-beta
INTRODUCTION
Orthodontic treatment is usually a very long treatment process due to patient compliance, the variety of cases, and the biomechanics used. This long treatment period is both the most important disadvantage of orthodontic treatment and the most important reason why patients avoid treatment. For this reason, there is great interest in accelerating tooth movement to shorten orthodontic treatment time by both clinicians and researchers.[1] Among these treatment approaches, rapid maxillary expansion (RME) is of great interest.[2] With RME, the duration of orthodontic treatment can be shortened, but the probability of relapse increases. Increasing bone regeneration in the inter-maxillary space may be beneficial to reduce the likelihood of relapse.[3-9]
Regarding this, researchers have conducted many studies to increase new bone regeneration in sutures after RME to shorten the retention period as well as total treatment time. Positive effects of treatments such as Vitamin D,[10] laser therapy,[11] transforming growth factor-β1 (TGF-β1),[12] curcumin and melatonin,[13] ozone application,[14] and intermittent compression[15] on suture bone formation have been reported.
Platelet-rich plasma (PRP), prepared from autologous plasma and containing concentrated platelets, is widely applied in cell-based therapies.[16] The alpha and dense granules of platelets contain more than 300 biologically active molecules that regulate the tissue regeneration process.[17] PRP is rich in vascular endothelial growth factor, TGF-β, tumor necrosis factor α, interferon, interleukin, and variable growth factors and cytokines. These molecules, which are released after the activation of platelets, play an important role in chemotaxis, mitogenesis, angiogenesis, osteoblastogenesis, osteoclastogenesis, differentiation, and wound healing processes.[18,19] PRP was first used in dentistry in 1998 in a mandibular reconstructive procedure by Robert Marx.[20] PRP has now been proven to be effective in oral surgeries such as periodontal therapy, implantology, tooth extraction, and regenerative dentistry because of its effects on bone regeneration.[21]
Distraction osteogenesis (DO) is related to osteogenesis and endogenous bone formation in the space between two separate bones, and it is especially used in the treatment of segmental bone defects.[22,23] The changes in the bone tissue in DO are histologically similar to the changes in the suture in RME. There are few studies examining the effects of PRP on DO. In previous studies, an increase in bone mineral density was detected by injecting PRP into DO’s subjects.[24,25] However, there are not many studies investigating the efficacy of PRP in RME. Our aim in this study was to examine the histological and radiological effects of PRP on bone tissue in the midpalatal suture in rabbits who underwent RME.
MATERIAL AND METHODS
Animals
This experimental study was carried out at Cumhuriyet University’s Faculty of Dentistry. Cumhuriyet University Experimental Animals Local Ethics Committee approved the study (dated 05/01/2012 and numbered 254). In the study, 24 male New Zealand rabbits, 14–18 weeks old and weighing 2.2–2.7 kg, were used. During the experiment, all animals were kept in separate cages at 22–24°C, 55–70% humidity, 12 h light/dark room conditions, and were fed ad libitum with ready-made pellets and had free access to water. For the rabbits to adapt to the laboratory environment, the rabbits were placed in their cages 2 weeks before the experiment and observed for their health. The oral hygiene status was periodically monitored to prevent plaque accumulation from causing various complications, such as nutritional issues and infection risks that may compromise trial standards. The subjects were randomly selected and divided into three groups. All subjects underwent RME for 7 days and then held in the retention period for 14 days. Physiological saline injection was applied to the control group (n = 8) on the 1st day of retention, and PRP was applied to Group I (n = 8) on the 1st day of retention. PRP was applied to Group II (n = 8) 24 h after the start of expansion and on the 1st day of retention, for a total of 2 times.
At the end of the 21-day experimental period, rabbits in all groups were sacrificed with 200 mg/kg sodium pentobarbital, and then, the upper jaws of the rabbits were dissected and placed in 10% formalin solution for fixation. Retention appliances were removed after the fixation process was completed to prevent the possibility of relapse in the maxillary suture.
Expansion appliance and experimental procedures
The appliances are made of 0.028” wire on millimeter paper, and a single winding twist is made with the middle tip of the young pliers to form the helix of the spring. The helix of the spring is 5 mm in diameter; the distance between the outer arms is 20 mm, and the distance between the arm and the helix is 22 mm. The outer arms of the appliance were adjusted to give 250 g of force when they were touched to the distal of the incisors.[4,26] Heat treatment was applied to the appliance to improve its mechanical properties.
A hole was drilled in the anteroposterior direction from the gingival papilla level of the incisors. The helices on the arms of the appliance were connected to the holes drilled in the teeth with the help of ligature wire. The appliance was applied externally to not prevent the feeding of the experimental animal and to not cause food retention. All applications were performed under anesthesia with a mixture of 50 mg/kg Ketamine-Hydrochloride (HCL) and 9 mg/kg Xylazine-HCL.
Opening of the midpalatal suture was expected for 7 days from the application of the expansion appliance and the oral and general health of the subjects were observed every day. At the end of the 7-day expansion period, it was observed that the teeth were spaced. After the expansion, the expansion appliances were removed and replaced with 0.016” × 0.016” square steel wire retention appliances, and the retention phase was started [Figure 1].
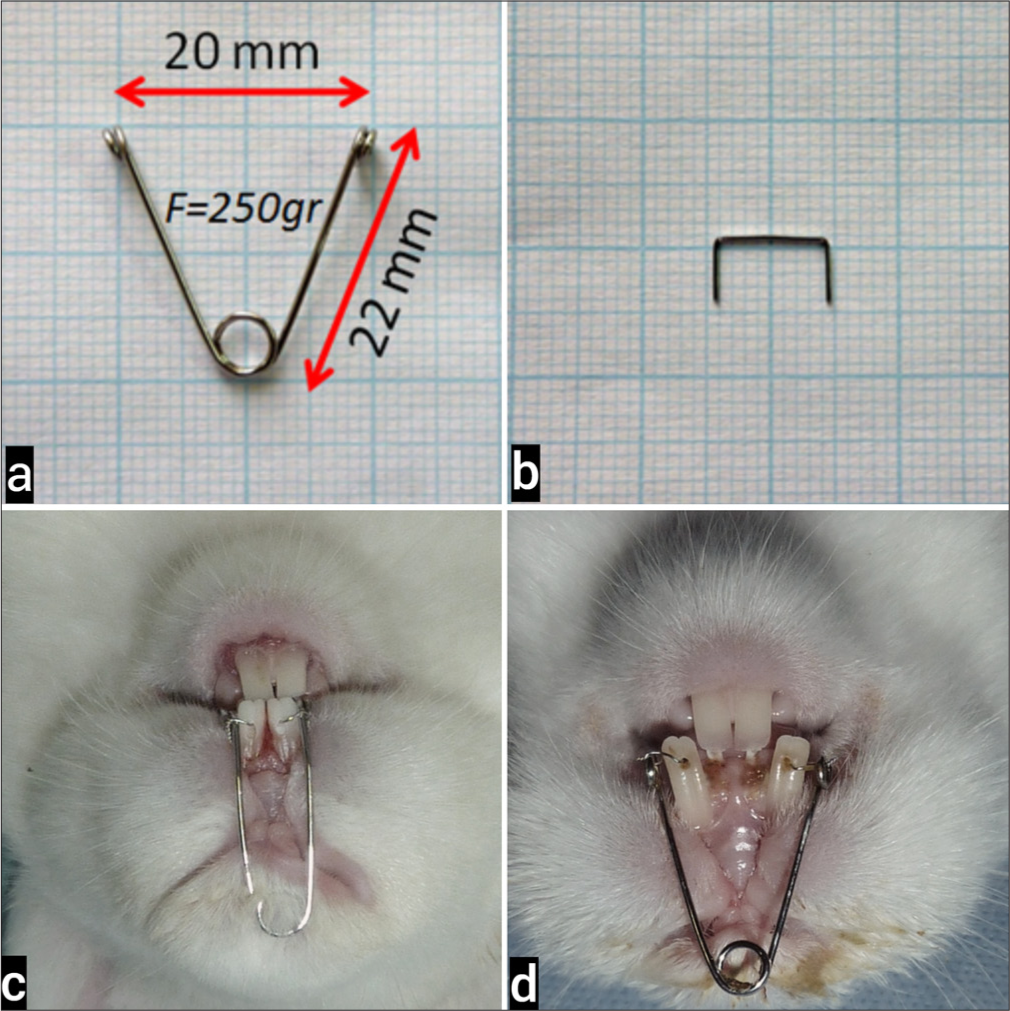
- (a) The expansion appliance was used in the experiment, (b) The retention appliance. (c) Extraoral application of the expansion appliance. (d) Midpalatal suture formation after 7 days of expansion.
PRP preparation and application
Rabbits were placed in the injection cage before PRP was prepared. Before the blood collection procedure, the marginal ear vein was dilated with the help of xylol. PRP preparation was done per the instructions of the manufacturer (PRP kit, Curasan, Pharma Gmbh AG, Kleinostheim, Germany). 8 mL of blood was drawn from the marginal ear vein of rabbits using a red butterfly needle set and a red monovette containing 0.5 mL of 10% trisodium citrate. The monovette was carefully inverted several times to mix the blood and the citrate completely; absolutely no shaking was done. After the blood collection procedure, the ears of the rabbits were rinsed with plenty of water, and Vaseline was applied. After the anticoagulant and blood were mixed homogeneously, the red monovette was placed in the centrifuge device and centrifuged at 2400 rpm for 10 min. At the end of the centrifugation process, two separate components, yellow in the upper part of the monovette and red in the lower part, were observed. The upper yellow part and the upper 1–2 mm of the red-colored layer were drawn into the yellow monovette, and approximately 4 mL of plasma obtained was centrifuged for the 2nd time. At the end of the second centrifugation process, which lasted for 15 min at 3600 rpm, the plasma was separated into two parts. The overlying platelet-poor plasma portion was removed. Approximately 0.7 mL of PRP remaining at the bottom was mixed in a vortex mixer for about 20 s for the activation of platelets, then transferred to the insulin injector and made ready for use. PRP application was performed under anesthesia using 50 mg/kg Ketamine-HCl and 9mg/kg Xylazine-HCl. The obtained PRP (0.7 mL) was injected locally into the suture from three different points along the suture from front anterior to posterior back to provide a homogeneous distribution.
Radiological Evaluation
In the study, multidetector computed tomography (MDCT) was used in the Department of Radiology, Faculty of Medicine, Cumhuriyet University to evaluate the new bone density formed after suture expansion. With MDCT, images were obtained at the beginning of the experiment (T0), after expansion (T1), and after retention (T2). Rabbits were placed in the gantry in the prone position under anesthesia in the MDCT device, and continuous axial sections of 0.5 mm thickness were taken. The sections taken were then examined on the monitors at the workstation through computer software. The obtained images were evaluated in axial, coronal, sagittal, and 3D dimensions, and the density of the new bone formed by the opening in the midpalatal suture was measured. The distance between the upper incisors was calculated by measuring from the mesial edges of the teeth at the gingival border. The radiologist performing the evaluation did not know about the study procedure and groups.
Histological and immunohistochemical evaluation
Tissue samples taken from experimental animals were fixed in 10% formalin for 24–48 h in accordance with the routine paraffin tissue follow-up protocol. Then, for the decalcification process, the samples were controlled by changing them every other day in ethylene diamino tetraacetic acid solution (dissolved in 100 mL 0.1 M phosphate buffer saline [PBS], pH: 7.1) by checking (for 4 weeks at + 4°C). Tissue samples that were cleared with xylene were embedded in paraffin after dehydration with a gradually increasing alcohol series. Sections of 5 µm thickness taken from the blocks were deparaffinized and rehydrated, stained with Hematoxylin-Eosin (H&E), and examined under a light microscope and evaluated. Sections of all groups were evaluated in terms of capillary dilatation, inflammatory cells, cell infiltration, osteoclasts, and osteoblasts in X400 magnification unit area (×40 objective ×10 ocular magnification: ×400). Osteoblast, osteoclast, and normal images of tissues under light microscope were evaluated as resting state. Multinucleated cells on the eosinophilic bone surface located in the Howship lacuna responsible for bone resorption in the groups were counted as osteoclasts, while areas containing blood elements and surrounded by endothelium were counted as capillary areas. The number of active osteoblasts was scored as 5–10 = 1, 11–20 = 2, > 21 = 3; the osteoclast count was scored as 1–2 = 1, 3–5 = 2, >6 = 3; and the number and width of capillaries were scored as normal = 1, moderately width and increased number = 2, and enlarged and increased number= 3.
Indirect immunohistochemical staining was performed on tissue sections using anti-bone morphogenetic protein-4 (BMP-4) and anti-TGF-β primary antibodies. Deparaffinized sections were first trypsinized and surrounded with Dako pap pen. Then, 3% hydrogen peroxidase was applied to inhibit the peroxidase activity in the tissue. Sections washed with PBS were incubated with non-immune blocking serum to provide specific staining. Sections were removed without washing the blocking solution. Sections for BMP-4 staining, sections were incubated with anti-BMP 4 (Mab1049 Chemicon International) primary antibody at 1:100 dilution at +4°C in humid environment for 18 h. Sections for TGF-β staining, it was incubated with anti-TGF-β (bs0086R BIOSS) primary antibody at 1:100 dilution at +4°C in humid environment for 18 h. Sections washed with PBS were incubated with biotin-labeled hydrogen peroxidase secondary antibody for 30 min. Then, sections washed with PBS were treated with streptavidin for 30 min. For the immunoreactivity to be visible, (3,3’-diaminobenzine)-applied sections were background stained with Mayer’s hematoxylin, passed through alcohol, cleared with xylene, and covered with Entellan. For negative control, slides were incubated in PBS instead of primary antibody. The immunostained sections were examined under a light microscope with an ×40 objective and scored as mild (1, +), moderate (2, ++), severe (3, +++), and very severe (4, ++++) according to the degree of immunoreactivity. The histologist performing the evaluation didn’t know about the study procedure and groups.
Statistical analysis
Expansion distance, bone density, and histological evaluation data obtained on the subjects at different measurement times were loaded into the Statistical Package for the Social Sciences (Version 14) program and statistical analyses were performed. The normality of continuous and categorical variable distributions was assessed using the Shapiro-Wilk test, and the homogeneity of variances was investigated using Levene’s test. Mean and standard deviation values of continuous variables and n and percentage values of categorical variables were given. The significance of the difference between the groups in terms of means was investigated by one-way analysis of variance, and the significance of the difference in terms of median values was investigated with the Kruskal–Wallis test. If the results of one-way analysis of variance or Kruskal–Wallis test statistics were found to be significant, the condition(s) causing the difference was determined using the post hoc Tukey Honestly Significant Difference or Mann–Whitney U test with Bonferroni correction. The significance of the difference in terms of the means of repeated measurements within the groups was evaluated using the dependent sample t-test when the number of repeated measurements was two and using the analysis of variance in repeated measurements in the evaluation of more than two repeated measurements. If the analysis of variance results were found to be significant in repeated measurements, the follow-up time(s) that caused the difference was determined using the Bonferroni corrected multiple comparison test. Unless otherwise stated, P < 0.05 was considered statistically significant, but Bonferroni correction was used to control for Type I errors in all possible multiple comparisons in this study.
RESULTS
There were no problems with the expansion procedure during the experiment. It was observed that the appliances fell off because five rabbits gnawed at the ligature wire. These appliances were reconnected on the same day. Apart from this, no periodontal problems, erythema, edema, or inflammation findings other than a slight hyperemia in the gums were observed during and after the application due to the appliance used in the subjects.
The comparison results of the distances between the teeth measured from the gingival level of the mesial edge of the maxillary incisors are given in [Table 1]. The MDCT images obtained in T1 and T2 periods are shown in [Figure 2]. There was no statistically significant difference between the groups in terms of mean enlargement amounts in the T1 period (P = 0.978). There was no statistically significant difference between the groups in terms of mean enlargement amounts in the T2 period (P = 0.899). The mean amount of expansion in expansion and retention in the control group was statistically similar (P = 0.229). The mean amount of expansion in expansion and retention in Group I was found to be statistically similar after Bonferroni correction (P = 0.043). The mean amount of expansion in expansion and retention in Group II was found to be statistically similar (P = 0.067).
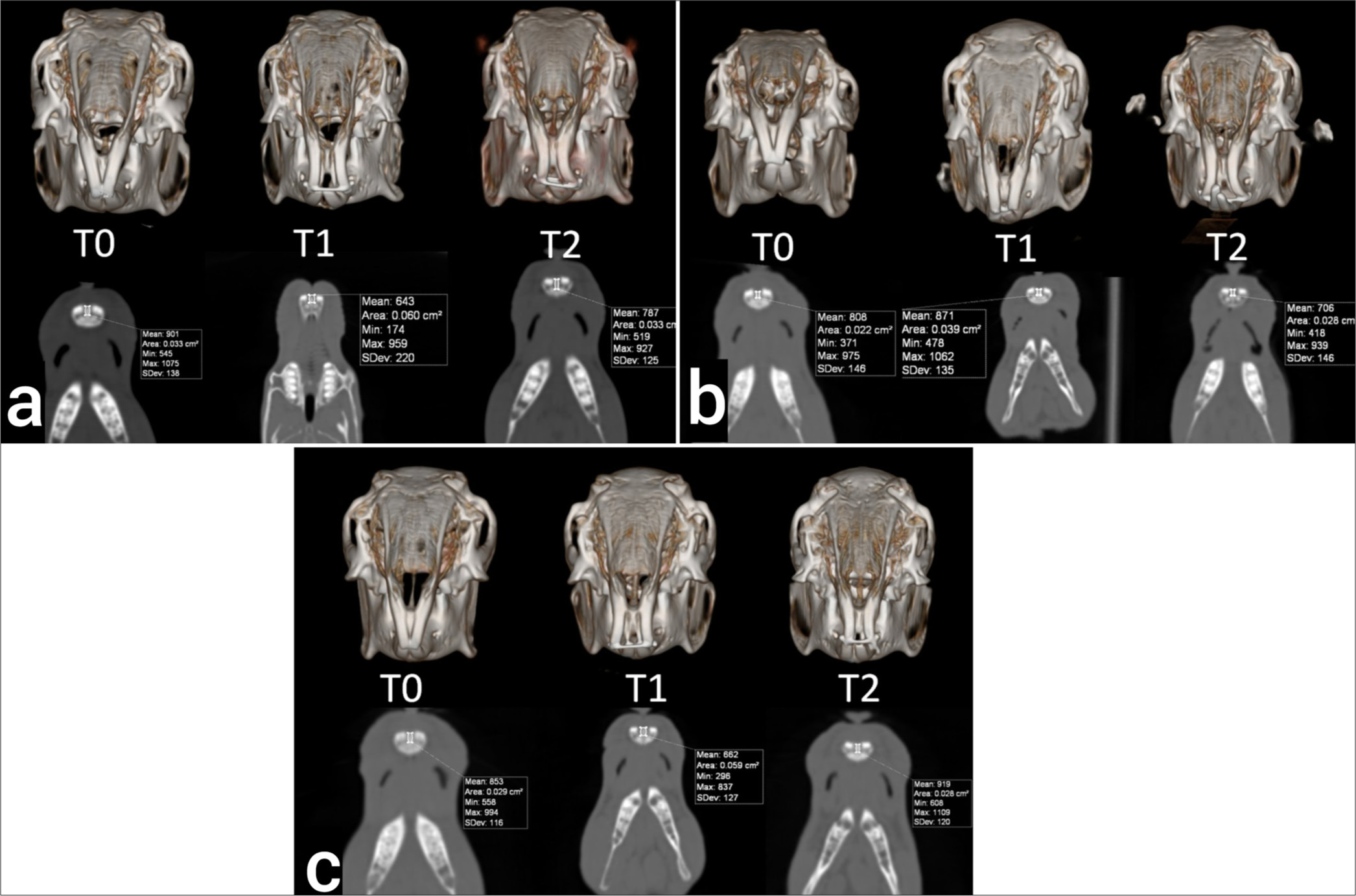
- Multidetector computed tomography images at T0, T1, and T2 periods. (a) Control, (b) Group I, (c) Group II.
| Groups | T1 | T2 | P-valuea |
|---|---|---|---|
| Control | 4.0±0.32 | 3.9±0.17 | 0.229 |
| Group I | 4.1±0.36 | 3.9±0.34 | 0.043 |
| Group II | 4.0±0.54 | 3.8±0.43 | 0.067 |
| P-valueb | 0.978 | 0.899 |
Bone density
Bone density values are shown in [Table 2]. In all groups, it was observed that the bone densities measured at T1 were significantly decreased compared to T0, and the bone densities measured at T2 were significantly increased compared to T1. While there was no difference between bone density measurements at T2 and T0 periods in both control and group I, bone density in T2 was significantly higher than T0 in Group II.
| Groups | T0 | T1 | T2 | P-valuea |
|---|---|---|---|---|
| Control | 776.7±58.20b | 660.0±51.77b,c | 760.0±50.60c | <0.001 |
| Group I | 763.2±111.08b | 658.8±104.29b,c | 803.7±88.64c | <0.001 |
| Group II | 774.2±59.20b,d | 700.3±66.10b,c | 898.3±58.49c,d | <0.001 |
The amount of bone density change in the T1-T0, T2-T0, and T2-T1 time intervals is given in [Table 3]. When the change in bone density in the T1 period compared to the T0 period was examined, a statistically significant difference was found between the groups (P = 0.009). Bone density statistically significantly decreased less in Group II compared to the control group (P = 0.009). There were no statistically significant differences between the control group and Group I and between Group I and Group II (P = 0.585 and P = 0.062). When the change in bone density in the T2 period compared to the T0 period was examined, a statistically significant difference was found between the groups (P<0.001). Bone density increased more in both Group I and Group II compared to the control group (P = 0.007 and P < 0.001). The increase in bone density in Group II was greater than in Group I (P < 0.001). When the change in bone density in the T2 period compared to the T1 period was examined, a statistically significant difference was found between the groups (P < 0.001). Bone density increased more in Group II compared to both the control group and Group I (P < 0.001 and P = 0.006). There was no statistically significant difference between the control group and Group I after Bonferroni Correction (P = 0.018). 3D and axial section images obtained by MDCT at T0, T1, and T2 periods are shown in [Figure 2].
| Variables | Control | Group I | Group II | P-valuea |
|---|---|---|---|---|
| T0-T1 | −116.7±13.66b | −104.3±23.14 | −73.8±25.14b | 0.009 |
| T0-T2 | −16.7±16.33b,c | 40.5±33.17c,d | 124.2±30.68b,d | <0.001 |
| T1-T2 | 100.0±12.65b | 144.8±30.51d | 198.0±27.90b,d | <0.001 |
Histological findings
In the H&E-stained sections obtained from the control group, the gingiva, teeth in both alveolar bone structures, periodontal ligament, and suture connective tissue were observed to be normal [Figure 3a]. While the normal histological structure was observed in the evaluation of the suture connective tissue, signs of inflammation, dilatation of blood capillaries, and cell infiltration were not observed. Osteoblasts with basophilic cytoplasm located in the endosteum, osteocytes in the lacunae, a small number of multinucleated osteoclasts with acidophilic cytoplasm located in the Howship lacuna, few and normal width capillaries, and a small number of inflammatory cells were observed.
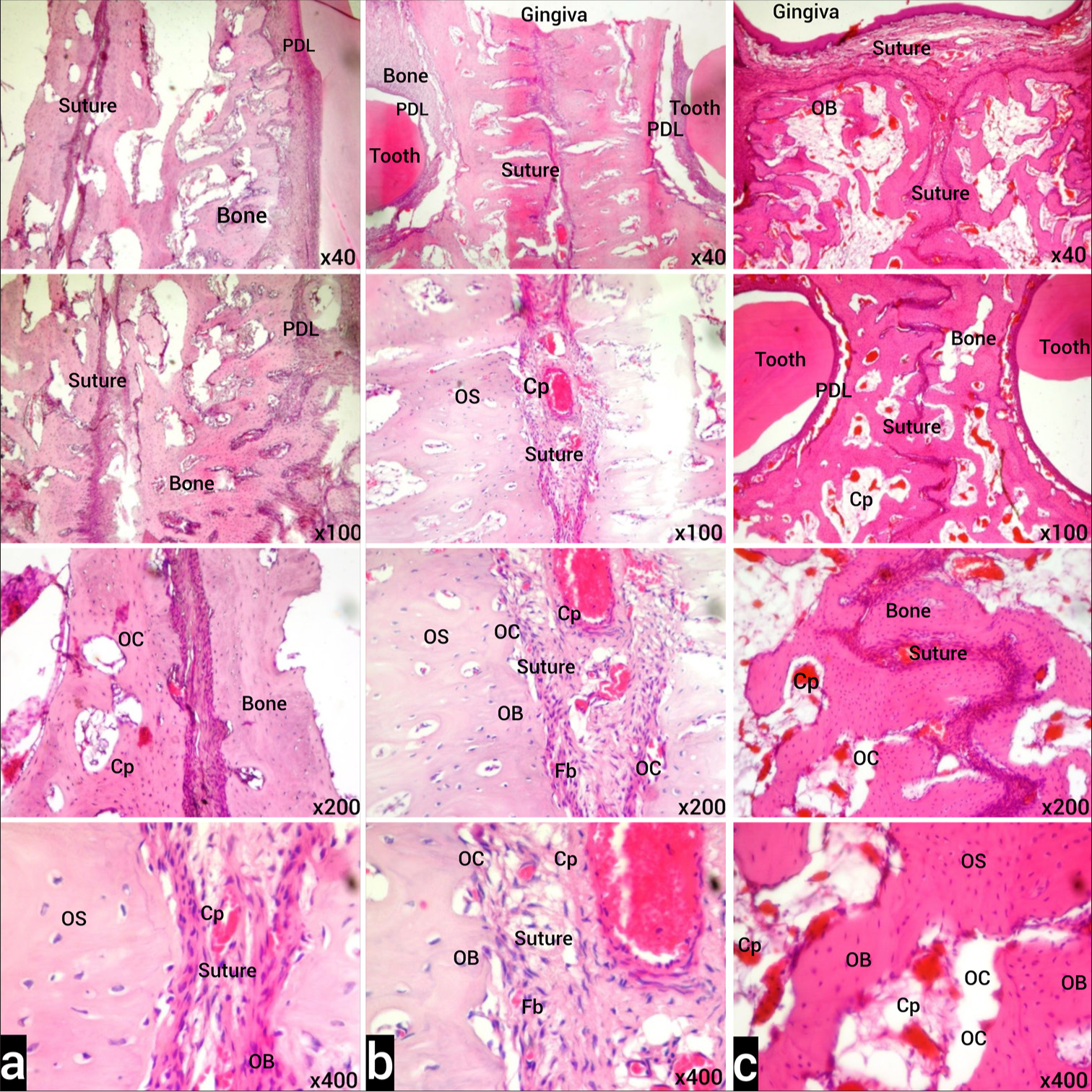
- (a) Images of H&E stained sections from the T2 period of the control group under light microscope. Normal histological appearance is observed in the examination of the tooth and its surrounding PDL, alveolar bone, and suture. (b) H&E-stained sections of tissue samples belonging to Group I in the T2 period under light microscope. Although less than Group II, compared to the control group, increased blood capillaries (Cp) in the examination of the tooth and surrounding PDL, alveolar bone and expanded suture, active fibroblasts (Fb), osteoclasts in the endosteum (OC), and active osteoblasts (OB) are observed. (c) Light microscope images of H&E-stained sections of tissue samples belonging to Group II in the T2 period. In the examination of the tooth and its surrounding PDL, alveolar bone and enlarged suture, increased blood Cp, active Fb, numerous OC, and active OB in the endosteum are observed. Osteoclast (OC), Osteoblast (OB), Osteocyte (OS), Capillary (Cp), Fibroblast (Fb) Stain: H&E (X40, X100, X200, X400 original magnifications). PDL: Periodontal ligament, H&E: Hematoxylin-Eosin.
In the sections obtained from Group I stained with H&E, there was an increase in blood vessels and dilatation and an increase in inflammatory cells in the connective tissue around the tooth compared to the control group, active osteoblasts and osteoclasts in the alveolar bone endosteum compared to the control group but less than in Group II [Figure 3b].
In the H&E-stained sections obtained from Group II, increased inflammatory cell activation, enlarged and increased number of capillaries in the connective tissue around the dental alveoli, increased number of multinucleated osteoclasts and active osteoblasts in the alveolar bone endosteum were observed. We observed that there were disruptions in the continuity of the collagen fibers forming the periodontal ligament and separations from place to place [Figure 3c].
Statistical analysis of histochemical and immunohistochemical [Figure 4] data obtained from the groups are given in [Table 4]. Inflammation, capillary dilatation, cell infiltration, osteoblastic activity, osteoclastic activity, and BMP-4 and TGF-β scores of Group II were significantly higher than the control group. Capillary dilatation, osteoblastic activity, and osteoclastic activity scores of Group I were significantly higher than the control group. No difference was observed between other parameters and groups.
| Control | Group I | Group II | P-valuea | |
|---|---|---|---|---|
| Inflammation | 1 (1-2)b | 2 (1-2) | 3 (2-3)b | 0.004 |
| Capillary dilation | 1 (1-2)b,c | 2 (2-3)c | 3 (2-3)b | 0.004 |
| Cell infiltration | 1 (1-2)b | 2 (1-2) | 3 (2-3)b | 0.005 |
| Osteoblastic activation | 1 (1-1)b,c | 2 (1-2)c | 3 (2-3)b | <0.001 |
| Osteoclastic activation | 1 (1-1)b,c | 2 (2-3)c | 3 (2-3)b | 0.002 |
| Anti-BMP-4 | 1 (1-2)b | 1.5 (1-2) | 2 (2-3)b | 0.020 |
| Anti-TGF-β | 1.5 (1-2)b | 2 (2-3) | 3 (2-3)b | 0.011 |
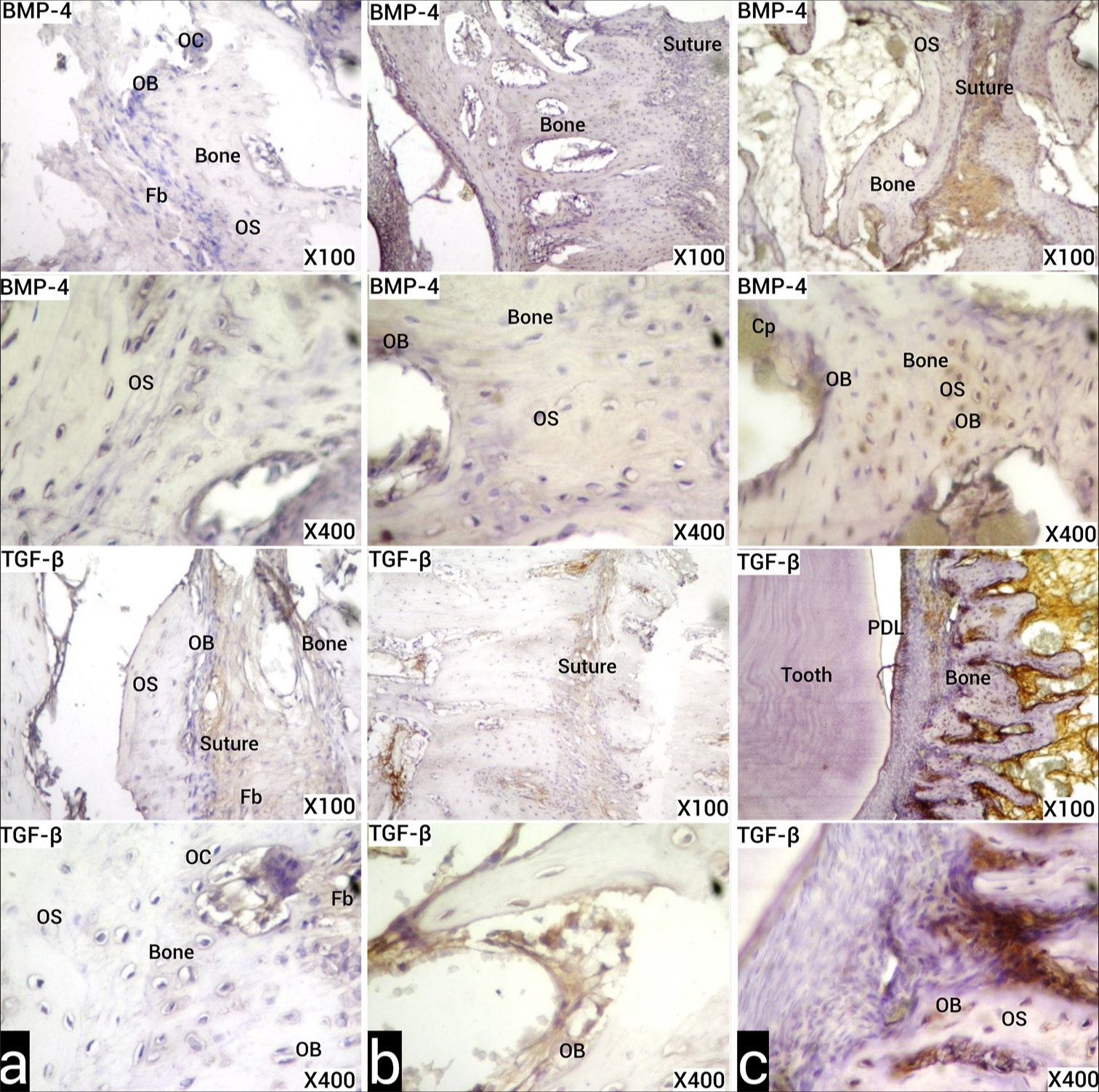
- (a) Images of the sections obtained from the experimental animals of the control group in the T2 period, stained with the indirect immunohistochemistry method with anti-BMP-4 and anti-TGF-β primary antibodies, under light microscope. Positive immunoreactivities are seen as stained brown. (b) Images of the samples obtained in the T2 period from experimental animals belonging to Group I, under the light microscope, stained with the indirect IHC method with anti-BMP-4 and anti-TGF-β primary antibodies. Brown positive and increased immunoreactivity is observed in suture-forming fibroblasts, osteoblasts, and osteocytes. (c) Images of the samples obtained in the T2 period from experimental animals belonging to Group II, sections stained with the indirect IHC method with anti-BMP-4 and anti-TGF-β primary antibodies under light microscope. More BMP-4 and TGF-β immunoreactivity is observed in suture-forming fibroblasts, osteoblasts, and osteocytes than Group I. Osteoclast (OC), Osteoblast (OB), Osteocyte (OS), Capillary (Cp), Fibroblast (Fb). (X100, X400 original magnifications). BMP-4: Bone morphogenetic protein-4, TGF-β: Transforming growth factor-beta.
DISCUSSION
In this study, we histologically and radiologically evaluated the results of autologous PRP application to rabbits who underwent RME. A decrease in bone density was observed in all groups after RME application, and an increase in bone density was observed in all groups after retention. The greatest improvement after retention was in the group that received two doses of PRP, and the bone density in this group was significantly higher than the baseline values. Osteoblast count, osteoclast count, and BMP-4 and TGF-β expression were observed to be higher in the group that received two doses of PRP.
Although RME is used in the treatment of class III malocclusion, dental crowding, and crossbites,[5] it has frequent relapses and relatively high failure rates. Saito and Shimizu[6] suggested that RME relapse is high due to insufficient bone regeneration in the intermaxillary region. Therefore, treatment modalities such as mesenchymal stem cell (MSC) injection,[7] curcumin and melatonin,[13] vitamin D analog,[10] local administration of bisphosphonates,[8] low-level laser therapy,[9] lithium,[27] and light-emitting diode phototherapy[28] have been applied to increase bone regeneration in the intermaxillary region.
PRP has recently been applied in dental procedures as well as in the field of regenerative medicine. The effects of PRP on orthodontic tooth movement have been investigated using both experimental animals and clinical studies. Gulec et al.[29] observed a reduction in alveolar bone density with both high and medium concentrations of PRP injection. In their study on dogs, Rashid et al.[30] observed many enlarged blood vessels in the periodontal tissues in the PRP group and found that the osteoblast, cementoblast, and osteoclast activities of the PRP group were higher than the control group. Another study in rabbits documented that PRP can significantly reduce the amount of relapse after the removal of orthodontic force.[31] Contrary to the above positive studies, Akbulut et al.[32] reported that osteoblast and osteoclast cell numbers, and tartrate-resistant acid phosphatase, alkaline phosphatase, and TGF-β expression did not change after PRP injection.
There are few studies examining the effect of PRP on new bone formation after DO. Kitoh et al.[33] showed that injection of MSC + PRP increased new bone formation in the distracted region of the human femur. Kinoshita et al.[34] observed increased bone formation in rabbit maxilla with MSC + PRP injection. Ebadifar et al.[35] determined that rats treated with PRP + MSC had higher bone density than rats treated with PRP or MSC alone. Karakayali et al.[24] determined that PRP increased bone remodeling and newly formed bone strength and had significantly higher bone mineral density in CT. Bou Assi et al.[36] reported the positive effect of PRP injection on bone healing after mandibular distraction in rabbits. Latalski et al.[37] documented that PRP injection increased patient satisfaction with better functional results in human tibia DO. On the contrary, Hernandez-Fernandez et al.[38] could not observe any histological or radiological evidence that PRP administration increases bone formation in the early stages of DO. Alomari et al.[39] determined that in 18 patients who underwent RME, horizontal and vertical bone loss was observed after RME and PRP did not reduce this bone loss. Zeitounlouian et al.[40] determined that PRF had no effect on alveolar bone remodeling. Differences between study results can be attributed to variation in the study population, administration protocol, and the allogeneic nature of PRP.
Relapse can occur in 45% of patients after RME.[41] To avoid relapse, retainers should be used until the bone density in the midpalatal suture has the appropriate mineral content.[42] Because the retainers actually fix the teeth, the palatal osteoid plates get closer even during the retention period. Therefore, accelerating suture bone formation may reduce retention time and the likelihood of recurrence. In our study, the effectiveness of PRP on RME was determined radiologically by MDCT and histologically. The decrease in bone density in the T1 period in group II was less than in the other groups. We think that the less loss of bone density in the T1 period in Group II is due to the PRP injection performed 24 h after the start of expansion. When the bone density change in the midpalatal suture was examined with the help of MDCT in the initial and post-retention periods, the highest bone density increase was detected in Group II, to which PRP was applied twice. These results of our study are in line with the results of the study by Al-Fakhry et al.,[43] which showed an increase in bone density in the cervical and medial regions of the alveolar bone after PRF injection. Supporting the MDCT findings, vascularization and osteoblast and osteoclast activation in histological sections in Group I, to which PRP was applied once, were significantly higher than the control group. We determined that inflammation, cell infiltration, osteoblastic activation, osteoclastic activation, and vascularization were more in Group II, to which PRP was applied twice. In our study, it was determined that the difference in incisor distance between the groups was insignificant in the T1 and T2 periods. This result actually shows that the expansion and reinforcement apparatus we used in our study were prepared as standard. The purpose of measuring the amount of expansion was to evaluate the effectiveness of the devices that provide expansion and retention. In our study, the amount of expansion was measured from the distance between the teeth but not from the distance between the bones. For these reasons, it is normal that there is no significant difference in the amount of expansion measured from the distance between the teeth. However, in our study, significant differences were found between groups in terms of MDCT and histological evaluation, bone density, and bone formation. BMP and TGF-β are the most important factors in bone formation and help maintain the balance of bone resorption and bone formation.[44] It is known that PRP is particularly rich in TGF-β.[20] In our study, BMP-4 and TGF-β were evaluated immunohistochemically. We determined that both BMP-4 and TGF-β expression were higher in group II rabbits than in the control rabbits. The reason for the improvement observed both radiologically and histologically in our study is that PRP has growth factors that increase both osteoclastic and osteoblastic activity. Moreover, PRP also achieves these effects through BMP-4 and TGF-β. The radiological and histological results of our study confirm the results of studies showing the positive effects of PRP on tooth movement.
Reports indicate that platelet degranulation and the release of growth factors occur within the initial 3–5 days, resulting in a growth factor activity duration of 7–10 days.[45] The immediate effects of the growth factors released during platelet degranulation, as well as the platelets themselves, are known to progressively decrease after the initial 5–6 days.[20] Nonetheless, given that the lifespan of active osteoblasts is approximately 3 months, it has been proposed that the physiological mechanism of bone repair will persist in advancing toward bone regeneration.[46] In our study aimed to examine the dose-dependent effects of PRP application at specific intervals, we believe that the increased positive effects observed in Group II emerged as a result of sustained release rather than cumulative effects.
A procedure that is conducted through the collection of blood may be regarded as overly invasive and therefore unsuitable for the purposes of retention, particularly in younger patients. However, the issue of relapse following RME is a concern for all patients, regardless of age. While the opening of the suture is a requisite criterion for the success of RME, it is equally important that the resulting skeletal enlargement is permanent. Managing these processes requires going through retention periods that can last up to 6 months. This situation can raise concerns about the length of the treatment period. In our view, a relatively simple blood collection procedure that contributes to this process and reduces patient burnout from long retention periods cannot be considered too invasive.
One of the challenges encountered in the experimental phase is the limitations of the clinical expansion procedure. In smaller animals like rabbits and rats, which possess distinct maxillary structures, the mechanics and applications for maxillary expansion differ. The restricted oral cavity opening in rabbits and rats, along with the placement of premolar and molar teeth in the deeper sections of the oral cavity, the diminutive size of the teeth, the short crown lengths, and the close contact between the teeth preclude the implementation of a banded appliance or the activation of a screw. For this reason, most current research focuses on experimental maxillary growth in the premaxilla region using rabbits and rats.[4-8,10-12] However, applying force to the upper incisors of rats or rabbits causes a rapid tipping, leading to the teeth dislodging from the alveolus.[26] Furthermore, holes opened near the gingiva shift to the incisal with the eruption of the teeth, causing an increase in tipping. As a result, the effect on the suture is relatively reduced.
CONCLUSION
The application of PRP has been demonstrated to exert a beneficial influence on the outcome of RME. Our findings indicate that while a single application of PRP resulted in increased bone formation, a repeated application (twice) demonstrated a more pronounced efficacy. It is hoped that this will have a beneficial impact on the stability of the expansion and the requisite retention time following expansion. Nevertheless, future randomized controlled clinical studies should concentrate on examining the impact of PRP injection into the expanded midpalatal suture at varying doses and over distinct time periods on expansion stability and retention time.
Ethical approval
The research/study was approved by the Institutional Review Board at Cumhuriyet University Experimental Animals Local Ethics Committee, approval number 254, dated 5th January, 2012.
Declaration of patient consent
Patient’s consent is not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship: This work is supported by the Scientific Research Project Fund of Cumhuriyet University under the Project number DİŞ-104.
References
- Effect of a submucosal injection of platelet-rich plasma on the rate of orthodontic tooth movement-a split-mouth, single-blind randomized controlled trial. Contemp Clin Dent. 2023;14:39-44.
- [CrossRef] [PubMed] [Google Scholar]
- New technique for midpalatal osteotomy in surgically-assisted rapid palatal expansion. Br J Oral Maxillofac Surg. 2017;55:556-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of rapid maxillary expansion on the midpalatal suture: A systematic review. Eur J Orthod. 2015;37:651-5.
- [CrossRef] [PubMed] [Google Scholar]
- Stimulation of bone formation by dietary boron in an orthopedically expanded suture in rabbits. Angle Orthod. 2009;79:984-90.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of mesenchymal stem cells injection and low-level laser therapy on bone formation after rapid maxillary expansion: An animal study. Am J Stem Cells. 2020;9:78-88.
- [Google Scholar]
- Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop. 1997;111:525-32.
- [CrossRef] [PubMed] [Google Scholar]
- Bone marrow mesenchymal stem cells enhance bone formation in orthodontically expanded maxillae in rats. Angle Orthod. 2015;85:394-9.
- [CrossRef] [Google Scholar]
- Effects of bisphosphonates on sutural bone formation and relapse: A histologic and immunohistochemical study. Am J Orthod Dentofacial Orthop. 2011;140:e31-41.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of laser therapy on patients who underwent rapid maxillary expansion; a systematic review. Lasers Med Sci. 2018;33:1387-95.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of ED-71, a new active vitamin D analog, on bone formation in an orthopedically expanded suture in rats. A histomorphometric study. Eur J Dent. 2009;3:165-72.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of single and multiple low-level laser applications after rapid palatal expansion on bone regeneration in rats. J Lasers Med Sci. 2020;11:S37-42.
- [CrossRef] [PubMed] [Google Scholar]
- Stimulation of bone formation in the expanding mid-palatal suture by transforming growth factor-beta 1 in the rat. Eur J Orthod. 1996;18:169-79.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of curcumin and melatonin on bone formation in orthopedically expanded suture in rats: A biochemical, histological and immunohistochemical study. Orthod Craniofac Res 2018:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of different concentrations of topical ozone administration on bone formation in orthopedically expanded suture in rats. Eur J Orthod. 2016;38:281-5.
- [CrossRef] [PubMed] [Google Scholar]
- Response of the expanded inter-premaxillary suture to intermittent compression. Early bone changes. Aust Orthod J. 2010;26:49-55.
- [CrossRef] [Google Scholar]
- Platelet-rich plasma and regenerative dentistry. Aust Dent J. 2020;65:131-42.
- [CrossRef] [PubMed] [Google Scholar]
- Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl 1):S13-33.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma: New performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin Oral Investig. 2020;24:569-84.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638-46.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich fibrin in bone regenerative strategies in orthodontics: A systematic review. Materials (Basel). 2020;13:1866.
- [CrossRef] [PubMed] [Google Scholar]
- Stem cells rejuvenate radiation-impaired vasculogenesis in murine distraction osteogenesis. Plast Reconstr Surg. 2015;135:799-806.
- [CrossRef] [PubMed] [Google Scholar]
- Applications of stem cells in orthodontics and dentofacial orthopedics: Current trends and future perspectives. World J Stem Cells. 2018;10:66-77.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of platelet-rich plasma on bone regenerate consolidation in distraction osteogenesis: An experimental study in rabbits. Acta Orthop Traumatol Turc. 2022;56:8-13.
- [CrossRef] [PubMed] [Google Scholar]
- Transplantation of autologous bone marrow mesenchymal stem cells with platelet-rich plasma accelerate distraction osteogenesis in a canine model. Cell J. 2015;17:243-52.
- [Google Scholar]
- Tissue response to the movement of bones. Am J Orthod. 1973;64:229-47.
- [CrossRef] [PubMed] [Google Scholar]
- Lithium delivery enhances bone growth during midpalatal expansion. J Dent Res. 2011;90:336-40.
- [CrossRef] [PubMed] [Google Scholar]
- Laser and LED phototherapy on midpalatal suture after rapid maxilla expansion: Raman and histological analysis. Lasers Med Sci. 2017;32:263-74.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of local platelet-rich plasma injection on the rate of orthodontic tooth movement in a rat model: A histomorphometric study. Am J Orthod Dentofacial Orthop. 2017;151:92-104.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of platelet-rich plasma on orthodontic tooth movement in dogs. Orthod Craniofac Res. 2017;20:102-10.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of platelet rich plasma injection on relapse of orthodontically moved teeth in rabbits. Egypt Orthod J. 2017;51:41-57.
- [CrossRef] [Google Scholar]
- Experimental investigation of effects of platelet-rich plasma on early phases of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2019;155:71-9.
- [CrossRef] [PubMed] [Google Scholar]
- Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis--a preliminary result of three cases. Bone. 2004;35:892-8.
- [CrossRef] [PubMed] [Google Scholar]
- Promoted new bone formation in maxillary distraction osteogenesis using a tissue-engineered osteogenic material. J Craniofac Surg. 2008;19:80-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of mesenchymal stem cells with platelet-rich plasma carriers on bone formation after rapid maxillary expansion: An Animal Study. Orthod Craniofac Res. 2022;25:151-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of autologous platelet-rich plasma on distraction osteogenesis in the mandible of rabbits: A morphologic and morphometric approach. J Biol Regul Homeost Agents. 2013;27:177-87.
- [Google Scholar]
- Enhancing bone healing during distraction osteogenesis with platelet-rich plasma. Injury. 2011;42:821-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of administration of platelet-rich plasma in early phases of distraction osteogenesis: An experimental study in an ovine femur model. Injury. 2013;44:901-7.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of injectable platelet-rich plasma in reducing alveolar bone resorption following rapid maxillary expansion: A cone-beam computed tomography assessment in a randomized split-mouth controlled trial. Angle Orthod. 2019;89:705-12.
- [CrossRef] [PubMed] [Google Scholar]
- Three-dimensional evaluation of the effects of injectable platelet rich fibrin (i-PRF) on alveolar bone and root length during orthodontic treatment: A randomized split mouth trial. BMC Oral Health. 2021;21:92.
- [CrossRef] [PubMed] [Google Scholar]
- Transverse dentoalveolar changes after slow maxillary expansion. Am J Orthod Dentofacial Orthop. 2011;140:317-25.
- [CrossRef] [PubMed] [Google Scholar]
- Bone density of the midpalatal suture 7 months after surgically assisted rapid palatal expansion in adults. Am J Orthod Dentofacial Orthop. 2011;139:S109-16.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of Injectable platelet rich fibrin (i-PRF) on reduction of relapse after orthodontic tooth movement: Rabbits model study. J Orthod Sci. 2022;11:10.
- [CrossRef] [PubMed] [Google Scholar]
- Osteocyte-intrinsic TGF-beta signaling regulates bone quality through perilacunar/canalicular remodeling. Cell Rep. 2017;21:2585-96.
- [CrossRef] [PubMed] [Google Scholar]
- Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin Oral Implants Res. 2005;16:349-56.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of PRP on autogenous sinus grafts. An experimental study on sheep. Clin Oral Implants Res. 2003;14:578-83.
- [CrossRef] [PubMed] [Google Scholar]







