Translate this page into:
Effect of low-intensity laser therapy on levels of biomarkers during orthodontic tooth movement – A systematic review

*Corresponding author: Wasundhara Ashok Bhad, Department of Orthodontics and Dentofacial Orthopedics, Government Dental College and Hospital, Nagpur, Maharashtra, India. wasundharabhad@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Arya R, Bhad WA, Chavan SJ. Effect of low-intensity laser therapy on levels of biomarkers during orthodontic tooth movement – A systematic review. APOS Trends Orthod. 2024;14:73-84. doi: 10.25259/APOS_190_2023
Abstract
This systematic review aimed to explore the association between low-intensity laser therapy (LILT) and various biomarkers, elucidating the potential of this therapeutic modality to expedite orthodontic tooth movement (OTM). A systematic literature search was conducted using electronic databases including PubMed, Central of the Cochrane Library, and Google Scholar databases. Randomized controlled trials (RCTs) and non-randomized controlled clinical trials (nRCTs) using LILT as an adjunct to the standard orthodontic procedures in human and animal subjects as participants were included in the study. The quality of the human and animal studies was assessed using the Revised Cochrane risk of bias tool and Systematic Review Centre for Laboratory Animal Experimentation’s (SYRCLE’s) risk of bias tool respectively. Animal studies revealed increased receptor activator of nuclear factor-kappa B (RANK)/RANK ligand (RANKL), matrix metalloproteinase-9 (MMP-9), and collagen type I expression. Human studies showed elevated interleukin-1-beta (IL-1β), interleukin-16 (IL-16) and RANKL levels, suggesting LILT influences biomarkers associated with bone resorption and connective tissue rearrangement. A high risk of bias was observed in all animal studies and 5 out of 6 human studies. The systematic review concluded that LILT emerges as a promising technique for enhancing orthodontic tooth movement, influencing key biomarkers linked to osteoclastic activity and collagen synthesis. However, the high risk of bias in animal and human studies emphasizes the need for further research to validate findings and optimize laser parameters for clinical benefits.
Keywords
Low-intensity laser therapy
Biomarkers
Orthodontic tooth movement
Semiconductor diode laser
Accelerated orthodontics
INTRODUCTION
Orthodontic therapy involves the application of external physical force to teeth,[1] leading to cellular-level changes in periodontal tissues, including bone resorption and deposition, and degeneration and rearrangement of the periodontal ligament (PDL).[2] These changes trigger the production and release of various substances that contribute to the bone remodeling process.[3] Prolonged orthodontic treatment duration is a significant obstacle in orthodontics, leading to psychosocial effects on patients, increased risk of caries, gingival recession, and root resorption. As a result, there is currently a focus on identifying treatment procedures that can shorten the treatment duration without compromising the outcome.[4] One such procedure is the use of low-intensity laser therapy (LILT) to accelerate orthodontic tooth movement (OTM). LILT is a safe and a minimally invasive technique that stimulates wound healing,[5,6] muscle relaxation,[7] immune system regulation,[5] fibroblast proliferation,[8,9] and nerve regeneration[10] due to its biostimulatory effects. Non-surgical and device-assisted therapies have also been used to biologically accelerate tooth movement. Cytokines in the gingival crevicular fluid (GCF) play a crucial role in bone resorption and deposition during orthodontic treatment.[11,12] GCF analysis is a non-invasive and simple method that allows for repeated sampling,[13] and recent studies have evaluated the biological aspects of OTM by identifying different biologic markers in GCF.[12,14,15]
Despite numerous studies exploring the effectiveness of LILT in orthodontics, no systematic review has analyzed the impact of LILT on biomarkers during OTM. To address the knowledge gap, this systematic review aims to explore the association between LILT and various biomarkers, elucidating the potential of this therapeutic modality to expedite OTM.
MATERIAL AND METHODS
Protocol and registration
Protocol of this systematic review was registered on National Institute for Health Research, Prospero international prospective register of systematic reviews (PROSPERO: registration number: CRD42022355520). The review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines 2020 and Cochrane Handbook for Systematic Reviews of Interventions version 6.3.
Eligibility criteria
Inclusion criteria
The inclusion criteria included the following:
Clinical trials (Randomized and Non-randomized) and Animal studies
Studies assessing the effect of LILT on levels of different biomarkers
Publications in English language, with full text available in soft or hard copy
Relevant studies published from 2000 to 2023.
Exclusion criteria
The exclusion criteria included the following:
Descriptive studies, review articles, opinion articles, and online published final dissertations
Studies dealing with medically compromised patients
Studies involving participants with periodontal problems, liver and bone diseases, growth abnormality, and participants with any drug history.
Information sources, search strategy, and selection process
To conduct a comprehensive and systematic review on the effect of LILT on levels of biomarkers during OTM, a broad range of electronic databases such as PubMed, Central of the Cochrane Library, and Google Scholar were searched from January 2000 to March 2023. A hand search was also undertaken in orthodontic journals. Unpublished literature was searched on ClinicalTrials.gov. In addition, reference lists of relevant studies, meta-analyses, systematic reviews, and other review articles were screened for potential inclusion.
Search Strategy: The search strategy was developed using relevant Medical Subject Headings (MeSH) terms, keywords, and Boolean operators “AND” and “or” combination. The following search terms and their combinations were used in the search strategy: “low-intensity laser,” “low-level laser,” “low-power laser,” “semiconductor diode laser,” “tooth movement,” “orthodontic tooth movement,” “accelerated tooth movement,” “accelerated orthodontic tooth movement,” “biomarkers,” “molecular biomarkers,” “transforming growth factor,” “TGF,” “matrix metalloproteinase,” “MMP,” “interleukin,” “IL-1,” “IL-6,” “prostaglandin,” “prostaglandin E2,” “Receptor activator of nuclear factor kappa B ligand,” “RANKL,” “Osteoprotegerin”, “OPG” with Boolean characters “AND,” and “OR” combination. The search strategy was adapted to the specific syntax and subject headings of each electronic database.
Selection process
The selection process followed the PRISMA guidelines. Two reviewers independently screened the titles and abstracts of all potentially relevant articles identified in the search to determine their eligibility for inclusion. Full-text articles of potentially eligible studies were assessed independently by two reviewers for inclusion in the systematic review. Any discrepancies between the reviewers were resolved through discussion or consultation with a third reviewer.
Data collection process and data items
Once the relevant studies were identified, data were extracted from each study using a pre-designed data extraction form. The data items extracted included the study design, name of author, year of publication, study design, age and sex of participants. Additionally, description of participants and grouping, type of tooth movement, description of intervention site, type of laser, wavelength of laser, power output were also extracted. Furthermore the total time of irradiation, frequency of LILT, biomarker analyzed, method of analyzing the biomarker, main outcome (level of biomarker), and additional outcome (rate of OTM) were other data items that were extracted.
Risk of bias (RoB) assessment in animal studies
RoB assessment for animal intervention studies done using Systematic Review Centre for Laboratory Animal Experimentation’s (SYRCLE’s) RoB tool.
RoB assessment in human studies
RoB assessment for human studies was done using Revised Cochrane RoB tool.
RESULTS
Study selection
Electronic screening of PubMed and Google scholar identified 32 articles. After adjusting the duplicates, 25 articles were scrutinized for inclusion in the study. Majority of them were excluded as they did not have relevant title and abstract, leaving 15 articles. Subsequent to excluding two review article and two descriptive studies, only 11 original articles remained, which were included in this systematic review. The PRISMA flowchart of the electronic database search is represented in [Figure 1].

- Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of study selection, n: Number of articles, *: records identified are total number of records across all databases, **: automation tools were not used, records were excluded by the reviewers.
Study characteristics
Animal studies
Participant selection
Five animal studies were included, with rats as the study population. Overall, 253 animals were studied to evaluate the effect of LILT on OTM and levels of biomarkers. General characteristics and grouping of these animals are described in [Table 1].
| S. No. | Study ID | Study design | Species/age/sex/weight | Description of participants and grouping | Type of tooth movement | Intervention site (LILT) |
|---|---|---|---|---|---|---|
| 1. | Kawasaki andShimizu (2000)[2] | Non-randomized controlled trial | Wistar rats; 6 weeks; males; mean weight 180±10 g |
n=48 Histochemical detection=24 rats Further divided into six groups of four rats as follows – Irradiation groups (day 0–1, day 0–2, and day 0–4) and, non-irradiation groups (day 0–1, day 0–2, day 0–4) |
Mesialization of maxillary left first molar; closed coil spring; and force of 10 g | Mesial, buccal, and palatal sides of gingiva in the area of upper left first molar |
| 2. | Yamaguchi et al. (2007)[18] | Non-randomized controlled trial | Wistar rats; 6 weeks; males; mean weight 180±10 g |
n=50 Laser irradiation group (E)=25 Non-irradiation group (C)=25 |
Mesialization of maxillary right first molar; closed coil spring; and force of 10 g | Mesial, buccal, and palatal sides of gingiva in the area of upper right first molar |
| 3. | Fujita et al. (2008)[19] |
Non-randomized controlled trial | Wistar rats; 6 weeks; males; mean weight 180±10 g |
n=75 Laser irradiation group (E)=25 Light-emitting diode group (C)=25 Non-irradiation group (C)=25 E=Experimental C=Control |
Mesialization of maxillary right first molar; closed coil spring; and force of 10 g | Mesial, buccal, and palatal sides of gingiva in the area of upper right first molar |
| 4. | Kim et al. (2010)[1] | Randomized controlled trial | Sprague-Dawley rats; 15 weeks; males; weight 300–350 g |
n=30 Laser irradiation group (E)=15 Non-irradiation group (C)=15 |
Tooth movement initiated by inserting an elastic rubber ligature between maxillary incisors; and force of 19.6±3.2 g | Three gingival regions of labial and palatal sides of maxillary incisors |
| 5. | Yamaguchi et al.(2010)[20] | Non-randomized controlled trial | Wister rats; 6 weeks; males; weight 180±10 g |
n=50 Laser irradiation group (E)=25 Non-irradiation group (C)=25 |
Mesialization of maxillary right first molar; closed coil spring; and force of 10 g | Mesial, buccal and palatal sides of gingiva in the area of upper right first molar |
LILT: Low-intensity laser therapy
Out of five studies, four studies were non-randomized controlled trials and one was a randomized controlled trial. All the studies evaluated the effect of LILT on the rate of OTM.
Description of the type of tooth movement and site of intervention is enlisted in [Table 1]. [Table 2] shows the laser parameters used as well as the biomarker analyzed,along with the frequency and method of sampling. [Table 3] shows the main and additional outcome obtained by each study.
| S. No. | Study ID | Type of laser | Wavelength | Power output/total time of irradiation | Frequency of laser treatment | Biomarker analyzed | Frequency of sampling | Method of analyzing |
|---|---|---|---|---|---|---|---|---|
| 1. | Kawasaki andShimizu (2000)[2] | Ga-Al-As | 830 nm | 100 mW 3 min/point | Day 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 | Tartrate-resistant acid phosphatase | Day 0, 1, 2, 4, 12 | Immunohistochemical staining |
| 2. | Yamaguchi et al. (2007)[18] |
Ga-Al-As | 810 nm | 100 mW 3 min/point | Day 0, 1, 2, 3, 4, 5, 6, 7 | M-CSF and c-fms | Day 1, 2, 3, 4, 7 | Immunohistochemical staining and RT-PCR |
| 3. | Fujita et al. (2008)[19] | Ga-Al-As | 810 nm | 100 mW 3 min/point | Day 0, 1, 2, 3, 4, 5, 6, 7 | RANK, RANKL and OPG | Day 1, 2, 3, 4, 7 | Immunohistochemical staining and RT-PCR |
| 4. | Kim et al. (2010)[1] |
Ga-Al-As | 808 nm | 96 mW 10 s/point | Day 1, 2, 3, 4, 5, 6, 7 | Fibronectin and collagen type-1 | Day 1, 2, 7, 14, 21 | Immunohistochemical staining |
| 5. | Yamaguchi et al. (2010)[20] |
Ga-Al-As | 810 nm | 100 mW 3 min/point | Day 0, 1, 2, 3, 4, 5, 6, 7 | MMP-9, Cathepsin-K and alpha (v) beta (3) integrin | Day 1, 2, 3, 4, 7 | Immunohistochemical staining |
M-CSF: Macrophage colony-stimulating factor, MMP: Matrix metalloproteinase, RANK: Receptor activator of nuclear factor kappa B, RANKL: RANK ligand, OPG: Osteoprotegerin, RT-PCR: Reverse transcriptase polymerase chain reaction.
| S. No. | Author | Main outcome – Level of biomarker | Additional outcome – Rate of tooth movement |
|---|---|---|---|
| 1. | Kawasaki andShimizu (2000)[2] | The amount of bone formation and rate of cellular proliferation in the tension side and the number of osteoclasts in the pressure side were all significantly increased in the irradiation group when compared with the non-irradiation group (P<0.01) | In the laser irradiation group, the amount of tooth movement was significantly greater (1.3-fold) than that of the non-irradiation group in the end of the experiment |
| 2. | Yamaguchi et al. (2007)[18] |
Cells positively stained with M-CSF and c-fms were found to be significantly increased in the irradiation group on days 2 and 3 as compared with the non-irradiation group. Further, c-fms expression in osteoclast precursor cells was detected at an early stage (days 2 and 3) in the irradiation group | In the irradiation group, the amount of tooth movement was significantly greater than that of the non-irradiation group at the end of the experiment |
| 3. | Fujita et al. (2008)[19] | Cells that showed positive immunoreactions to the primary antibodies of RANKL and RANK were significantly increased in the irradiation group on day 2 and 3, compared with the non-irradiation group. In contrast, the expression of OPG was not changed. Further, RANK expression in osteoclast precursor cells was detected at an early stage (day 2 and 3) in the irradiation group | The amount of tooth movement was significantly greater than in the non-irradiation group by the end of the experimental period. |
| 4. | Kim et al. (2010)[1] | The immunohistochemistry results showed that the expression of fibronectin and collagen type I in the experimental group had significantly increased from day 1, with a more even distribution than in the control group, and that this difference was maintained until the end of the experiment. | There was no difference between the two groups in the amount of tooth movement. |
| 5. | Yamaguchi et al. (2010)[20] | Cells positively stained with TRAP, MMP-9, cathepsin K, and integrin subunits of a(v)b3 were found to be significantly increased in the irradiated group on days 2–7 compared with those in the non-irradiated group (P<0.05) | Amount of tooth movement was significantly greater in irradiated group than that in the non-irradiated group at the end of the experiment (P<0.05) |
M-CSF: Macrophage colony-stimulating factor, TRAP: Tartrate resistant acid phosphatase, MMP-9: Matrix metalloproteinase, RANK: Receptor activator of nuclear factor-kappa B, RANKL: RANK ligand.
Human studies
Participant selection
Six human studies were assessed in this systematic review, with a total population of 70 healthy participants. Except for one study,[16] all other studies specified the sex of the participants,and among them only one study[17] included female participants exclusively, whereas the rest of them included both male and female participants. The general characteristics of all the participants are mentioned in [Table 4] along with their distribution into different groups.
| S No. | Study design | Age/sex | Description of participants and grouping | Type of tooth movement | Intervention site (LILT) |
|---|---|---|---|---|---|
| 1. | Randomized split-mouth controlled trial | 11 females, mean age 19±4.21 years |
n=11 Left and right halves of the upper arches were randomly assigned to the laser group (E) and control group (C) |
Canine retraction | Total six irradiations Three on buccal side and three on palatal side of experimental canine • One on cervical third of root • One on apical third of root • One on middle third of root |
| 2. | Randomized split-mouth controlled trial | Eight females, seven males, mean age 16.2±1.32 years |
n=15 Left and right halves of the upper arches were randomly assigned to the laser group (E) and control group (C) |
Canine retraction | Total ten irradiations Five on buccal side and five on palatal side of experimental canine • Two on cervical third of root • Two on apical third of root • One on middle third |
| 3. | Split-mouth study | Not specified |
n=12 Laser irradiation on right upper quadrant (E) No irradiation on left upper quadrant (C) E=Experimental side C=Control side |
En masse retraction | Total ten irradiations Five on buccal side and five on palatal side of canine, lateral incisor, and central incisor • Two on cervical third of root • Two on apical third of root • One on middle third |
| 4. | Randomized split-mouth controlled trial | Six females, four males, aged 14–25 years |
n=10 Experimental side was assigned by lottery method with a sealed envelope (E) Other side acted as control (C) |
Canine retraction | Total ten irradiations Five on buccal side and five on palatal side of experimental canine • Two on cervical third of root • Two on apical third of root • One on middle third |
| 5. | Randomized split-mouth controlled trial | Seven females, three males Age-not specified |
n=10 Experimental side was assigned by lottery method with a sealed envelope (E) Other side acted as control (C) |
Canine retraction | Total ten irradiations Five on buccal side and five on palatal side of experimental canine • Two on cervical third of root • One on middle third and two on apical third of root |
| 6. | Randomized split-mouth controlled trial | Eight females, four males Aged 18–28 years |
n=12 Left and right halves of the upper arches were randomly assigned to the laser group (E) and control group (C) using coin toss method |
Canine retraction | Total four irradiations on experimental canine • Mesial buccal • Distal buccal • Mesial lingual • Distal lingual |
LILT: Low-intensity laser therapy.
Description of the type of tooth movement and site of intervention is enlisted in [Table 4]. [Table 5] shows the laser parameters used as well as the biomarker analyzed, along with the frequency and method of sampling. [Table 6] shows the main and additional outcomes obtained by each study.
| S. No. | Study ID | Type of laser | Wavelength | Power output/total time of irradiation | Frequency of laser treatment | Biomarker analyzed | Method of analyzing |
|---|---|---|---|---|---|---|---|
| 1. | Yassaei et al. (2016)[17] |
Ga-Al-As | 980 nm | 100 mW 10 s for cervical and middle third and 8 s for apical third |
Days 0, 7, 14, 21, and 28 every month | IL-6 | Quantitative analysis for measuring IL-6 concentration using available immunoassay kit (eBioscience, Ltd., Ireland, UK) |
| 2. | Üretürk et al. (2017)[21] |
Ga-Al-As | 820 nm | 20 mW 10 s/point | Day 0, the 3rd, 7th, 14th, 21th, 30th, 33rd, 37th, 44th, 51st, 60th, 63rd, 67th, 74th, 81st, 84th, 90thdays | IL-1ß and TGF-ß1 in GCF | Quantitative analysis using commercially available IL-1β and TGF-β1 ELISA test (ELISA, YH Biosearch Laboratory, Shanghai, China) |
| 3. | Jose et al. (2018)[16] | Ga-Al-As | 810 nm | 100 mW 10 s/point | Not specified | IL-1 β and PGE2 in GCF | Quantitative analysis using commercially available ELISA test (Raybiotech®Human IL1 β and Human PGE2) |
| 4. | Varella et al. (2018)[3] |
Ga-Al-As | 940 nm | 100 mW 10 s/point | For 3 consecutive days at the following intervals: • Start of canine retraction • 4 weeks later • 8 weeks later |
IL-1 β in GCF | Quantitative analysis using commercially available human IL-1 β ELISA kit (Krishgen BioSystems, Brea, Calif) |
| 5. | Jivrajani and Bhad Patil (2020)[22] | Ga-Al-As | 980 nm | 0.3 W 3 s/point | 1, 3, 7, and 14-day intervals in the 1stmonth. Thereafter on every 15 days till the complete canine retraction on the experimental side | MMP-9 in GCF | Quantitative analysis using ELISA kit for Human MMP 9 (RayBio®, RayBiotech, Inc., USA, Cat#: ELH-MMP9) |
| 6. | Zheng and Yang (2021)[23] | Semiconductor diode laser | 810 nm | 100 mW 40 s/point | Day 0, 7, 14, 21 | IL-1β, RANKL, and OPG in GCF | Quantitative analysis using human IL-1β ELISA kit (R and D Systems, Minneapolis, MN, US), the human OPG ELISA kit (R and D Systems), and the human RANKL ELISA kit (R and D Systems) |
TGF-β1: Transforming growth factor-Beta 1, IL-1β: Interleukin-1beta, GCF: Gingival crevicular fluid, PGE2: Prostaglandin E2, MMP: Matrix metalloproteinase, RANKL: RANK ligand, ELISA: Enzyme-linked immunosorbent assay, OPG: Osteoprotegerin.
| S. No. | Author | Main outcome – Level of biomarker | Additional outcome – Rate of tooth movement |
|---|---|---|---|
| 1. | Yassaei et al. (2016)[17] | No significant difference in the mean concentration of IL-6 at various stages of the treatment between both the groups during canine distalization (P>0.05). | Although the mean rate of canine retraction was higher in the experimental group (0.013 mm/day) than the control group (0.012 mm/day) and there was definitely a tendency for more canine retraction in the LLLI, but the results failed to show any significant difference between the mean rate of canine retraction of both groups (P=0.068). |
| 2. | Üretürk et al. (2017)[21] | 1stday IL-1ß levels were statistically higher than initial and 21stday levels (P=0.003, P=0.012). The rise in IL-1ß levels caused the negative correlations between 7thday IL-1β and 21stday TGF-β1 levels describes the tissue effects of laser application. | Amount of tooth movement in the laser group was 40% more than the control group. |
| 3. | Jose et al. (2018)[16] | Levels of IL1 β and PGE2 peaked at 7thday after LLLT and 21stday before LLLT from baseline. | LLLT assisted retraction was significantly faster than conventional retraction. |
| 4. | Varella et al. (2018)[3] | Increased levels of IL-1 β were observed in the experimental canines compared with the control canines (P<0.001). A positive correlation existed between the IL-1 β levels and the amounts of tooth movement across all time intervals. |
Cumulative tooth movements over 8-weeks were greater for experimental canines as seen on occlusogram, that is, 4.450 and 4.4903 mm, manually and digitally, respectively, compared with the control canines, that is, 2.025 and 2.0501 mm, manually and digitally, respectively. |
| 5. | Jivrajani and Bhad Patil (2020)[22] | MMP-9 concentration was high on the experimental side at 3 months. At the end of canine retraction (4.5 months), MMP-9 concentration was the same in both experimental and control group. | The average increase in rate of tooth movement on experimental side at 3 months was 44%. At the end of canine retraction (4.5 months) in the experimental group, the average rate increase was 38%. |
| 6. | Zheng and Yang (2021)[23] | Laser group had significantly higher IL-1β levels compared to the control group on days 21 and 28. Minimum OPG value was observed on day 7 in the laser group and on day 14 in the control group. On day 7, the OPG concentrations were significantly lower in the laser group than in the control group. RANKL level in the laser group was significantly higher than baseline on day 21; in contrast, RANKL levels remained practically constant in the control group. | At the end of 4 weeks of retraction, the canines were retracted 1.15±0.29 mm on the laser side and 0.85±0.23 mm on the control side. The cumulative tooth movement over 28 days was significantly higher in the laser group than in the control group. |
TGF-β1: Transforming growth factor-Beta 1, IL-1β: Interleukin-1beta, PGE2: Prostaglandin E2, MMP: Matrix metalloproteinase, LLLT: Low level laser therapy, RANKL: RANK ligand, OPG: Osteoprotegerin, LLLI: Low-level laser irradiation, OPG: Osteoprotegerin.
RoB
The RoB assessment for animal studies is summarized in [Figures 2 and 3] and for human studies in [Figures 4 and 5]. On overall assessment, all animal studies[1,2,18-20] had high RoB and out of six human studies, one study had low RoB[3] with remaining five having high RoB[16,17,21-23]

- Risk of bias summary for animal studies.
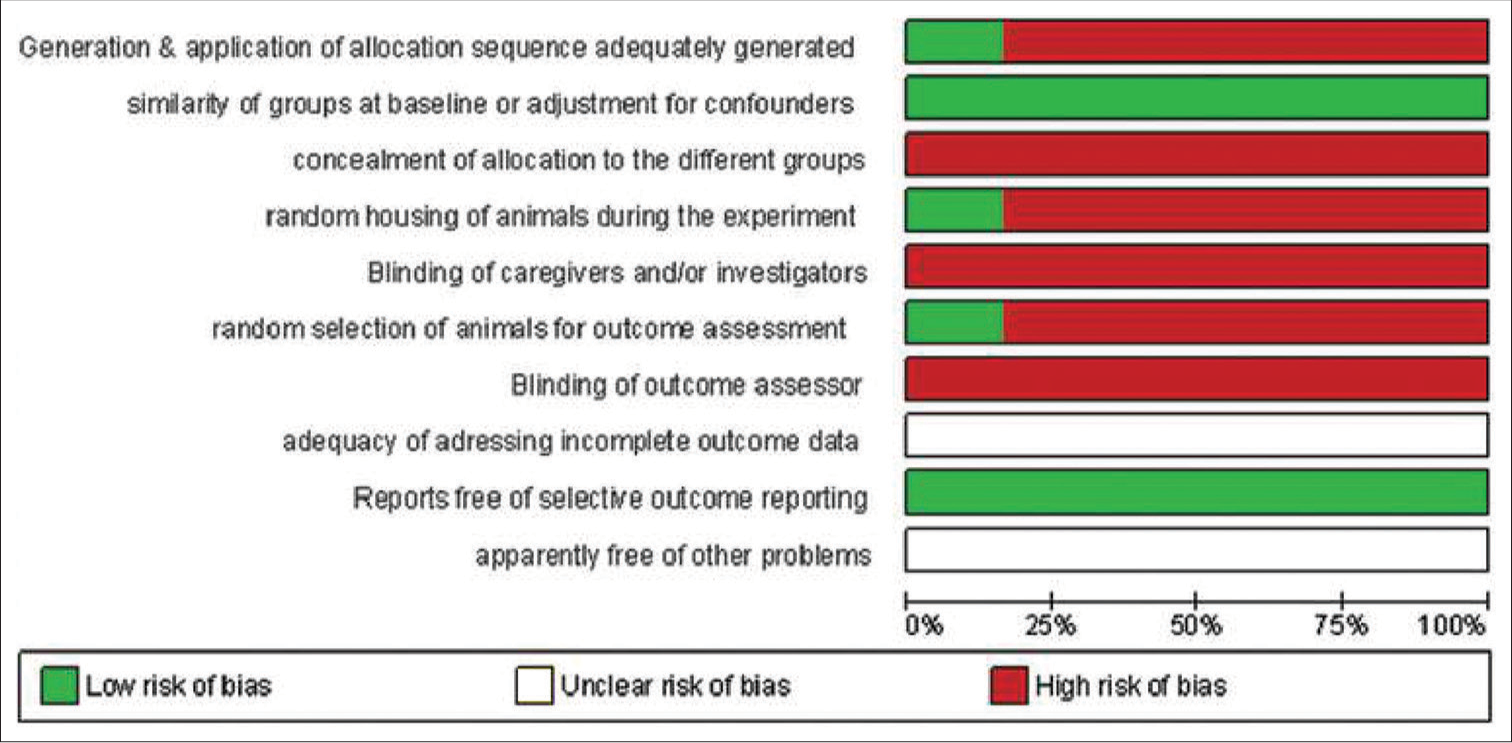
- Risk of bias graph for animal studies.
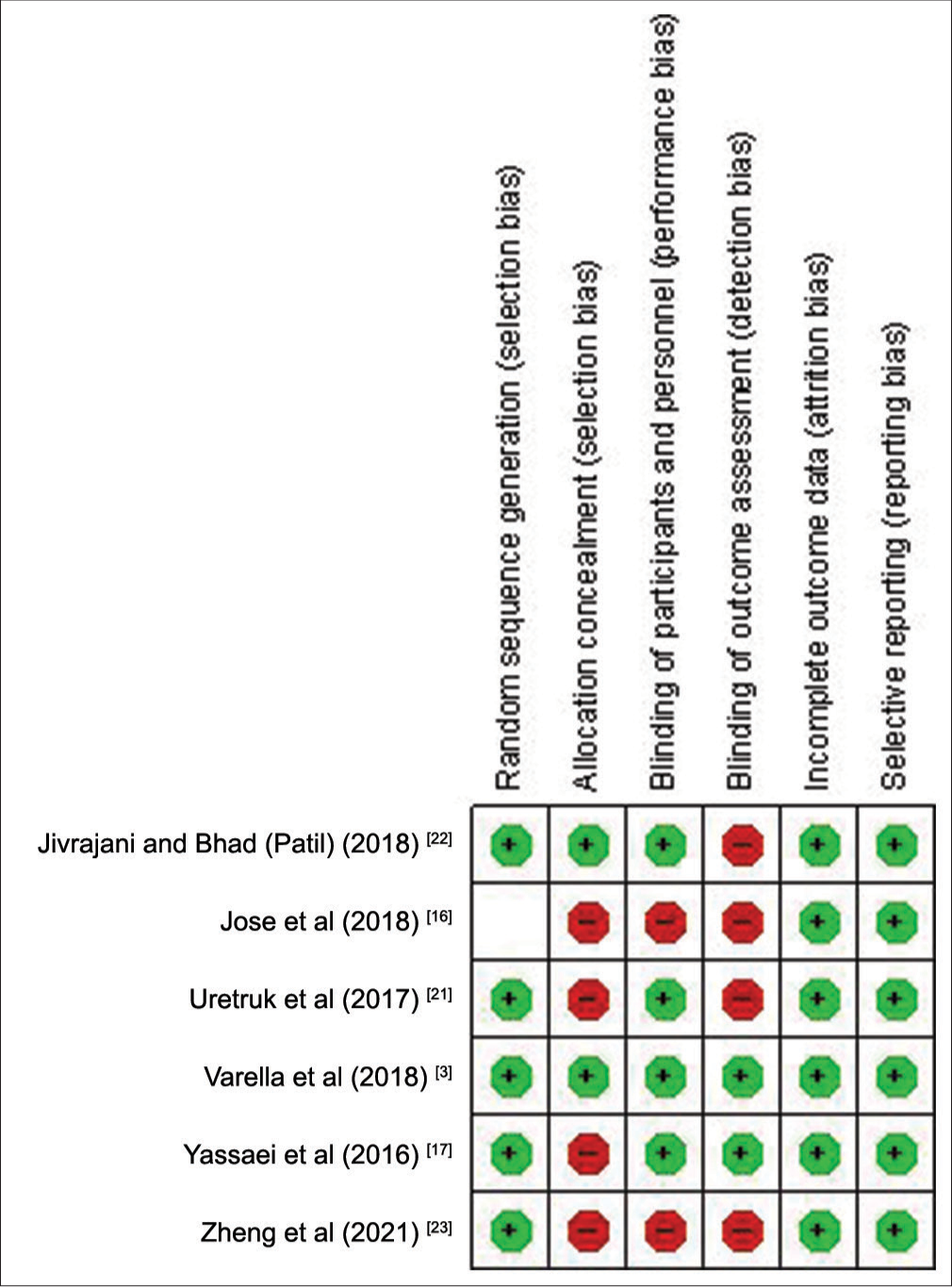
- Risk of bias summary for human studies.
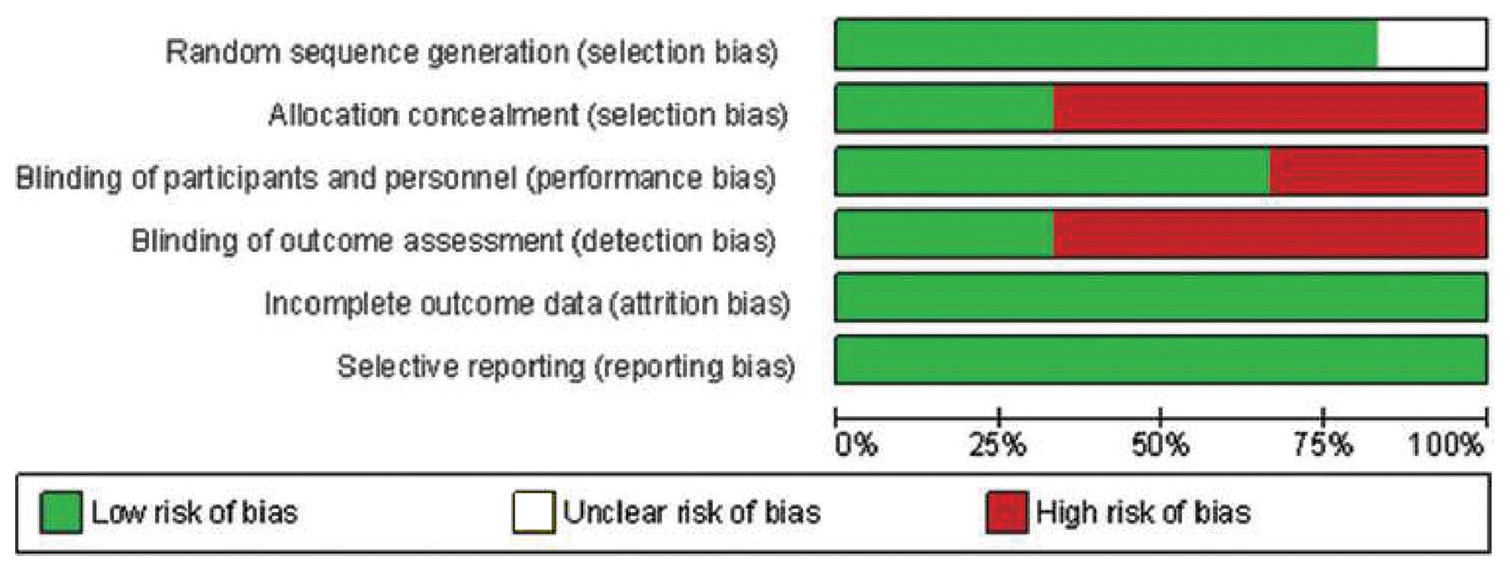
- Risk of bias graph for human studies.
DISCUSSION
The primary purpose of this systematic review was to summarize the evidence from multiple clinical trials that have compared the effect of LILT on levels of different biomarkers during OTM. Thorough screening of the literature yielded 11 articles including six human and five animal studies.
Animal studies
Out of the five animal studies analyzed in this systematic review, four of them concluded that LILT led to an acceleration in tooth movement.[2,18-20] However, one study reported that there was no significant difference in the rate of tooth movement between the experimental group and the control group.[1]
Kawasaki and Shimizu[2] found that low-energy laser irradiation during experimental tooth movement in rats increased the amount of tooth movement and osteoclast formation on the pressure side. They used the expression of tartrate resistant acid phosphatase as a lineage marker to identify the macrophage/osteoclast lineage.[2]
Fujita et al.[18] and Yamaguchi et al.[19] showed that low-energy laser irradiation increased the velocity of tooth movement by promoting the expressions of receptor activator of nuclear factor-kappa B (RANK)/RANK ligand (RANKL) and macrophage colony-stimulating factor/colony-stimulating factor-1 receptor, respectively, which are essential for osteoclastogenesis and bone resorption. These factors are necessary and sufficient for bone resorption, which is the rate-limiting step in OTM.[18,19]
Yamaguchi et al.[20] conducted a study on the impact of LILT on OTMs in rats, focusing on the roles of matrix metalloproteinase (MMP)-9, cathepsin K, and alpha(v) beta (3) integrin. Their research indicated that MMP-9 is involved in the invasive activity of osteoclasts, cathepsin K plays a critical part in the degradation of the bone organic matrix through osteoclasts, and alpha(v) beta (3) integrin is responsible for the tight attachment of osteoclasts to the bone matrix. They observed higher expression of MMP-9, cathepsin K, and alpha(v) beta (3) integrin on the pressure side, as well as a significant increase in the velocity of OTM on the laser-treated side.[20]
In their research on rats, Kim et al.[1] determined that the experimental group exhibited a significant increase in the expression of fibronectin and collagen type I from day 1, with a more uniform distribution compared to the control group. These differences were maintained until the conclusion of the study. Based on these findings, the authors concluded that LILT aids in the rearrangement of connective tissues during tooth movement in rats.[1]
However, in all these studies, animals were sacrificed within one or two weeks to obtain tissue samples, which prevented the determination of the long-term effects of the laser treatment. Therefore, it was necessary to conduct studies on humans to assess the changes in biomarker levels over a longer period during orthodontic treatment with LILT.
Human studies
Out of the six human studies analyzed in this systematic review, five studies[3,16,21-23] concluded that LILT resulted in a higher rate of tooth movement. However, one study[17] did not find a significant difference in the average rate of tooth movement between the experimental and control group.
Interleukin-1beta (IL-1β) is a powerful cytokine that promotes osteoclast activity and is produced in sufficient quantities by the PDL to diffuse into the GCF. IL-1β is considered a biomarker of OTM.[3] Üretürk et al.[21] found that low-level laser therapy (LILT) had a significant effect on the levels of IL-1β in GCF. Twenty-four hours after LILT, there was a significant difference in IL-1β levels between the laser and control groups, but only at the site of compression due to increased osteoclastic activity in that area.[21] According to Jose et al. and Zheng and Yang, IL-1β levels peaked on day 7 after LILT, and the laser group had significantly higher levels on day 21.[16,23] All these observations were attributed to the role of IL-1β in bone resorption, which is the limiting factor in OTM.
Varella et al.[3] demonstrated a four-fold increase in IL-1β levels after four weeks and a ten-fold increase after eight weeks in patients who received continuous laser irradiation compared to those who did not receive LILT. This finding suggests that the accumulation of IL-1β in response to laser therapy may increase over time, possibly due to the extended application of laser irradiation during canine retraction.[3]
The production of osteoclast precursors and osteoclast proliferation is stimulated by inflammatory cytokines like IL-6, which also play a crucial role in regulating bone remodeling activity in specific regions and initiating the acute inflammatory response at the onset of orthodontic treatment.[17] Yassaei et al.[17] conducted a study on the effect of LILT on the level of IL-6 in GCF and determined that there was no significant difference in the average concentration of IL-6 during various stages of canine distalization treatment between the groups. However, this result could be because the second GCF sample was collected six months after stage 0, whereas other studies have examined the increase in cytokine levels over shorter periods of time, such as 24 h or 1 week.[17]
Osteoclasts produce MMP-9, which is primarily a marker for bone resorption.[20] This marker can be analyzed on the pressure side during tooth movement. Jivrajani and Bhad Patil[22] studied the impact of LILT on MMP9 levels in GCF and found that LILT increased MMP-9 expression in GCF during the initial three months of canine retraction. However, this effect declined after three months as there was no significant difference in the change of MMP-9 concentration between the experimental and control groups.[22] This result suggests that LILT may be more effective in the early stages of OTM, as demonstrated by previous studies conducted by Doshi-Mehta and Bhad-Patil,[24] and Saito and Shimizu.[25]
RANKL plays a crucial role in the formation of osteoclasts, which are responsible for bone resorption.
When RANKL binds to its receptor, RANK, it triggers a series of internal signaling events that ultimately lead to the breakdown of bone tissue. Osteoprotegerin (OPG), a soluble decoy receptor, inhibits this interaction. During the early stages of OTM, the previous clinical studies have found that OPG levels tend to remain the same or decrease while RANKL levels tend to increase.[22,23] However, Zheng and Yang[23] recently investigated the impact of LILT on the levels of RANKL and OPG in GCF and found that the RANKL level was significantly higher at day 21 in the laser group, while it remained constant in the control group. This is because the binding of RANKL to RANK initiates bone resorption, which is prevented by OPG.
Several studies have pointed out the interaction between biomarkers. Üretürk et al.[21] investigated how LILT affects the levels of IL-1β and transforming growth factor-beta 1 (TGF-β1) in GCF. Although IL-1β levels exhibited a significant difference between the laser and control groups at 24 h only in the compression site due to increased osteoclastic activity in the same area, TGF-β1 levels showed no significant difference between the two groups, possibly due to reduced gene and protein expression of TGF-β1 in cells exposed to IL-1β.[21] In contrast, Jose et al. also studied IL-1β and prostaglandin E2 (PGE2) in GCF and concluded that the levels of both IL-1β and PGE2 reached their peak at the 7 and 21 day after LILT, indicating that PGE2 was upregulated by IL-1β.[16] The GCF collection protocol recommended by Jivrajani and Bhad Patil[22] offers valuable insights for future research, particularly in exploring the prolonged effects of LILT on biomarker levels and the rate of OTM.
CONCLUSION
Overall, the studies suggest that LILT enhances OTM by modulating osteoclast activity and collagen synthesis. However, the studies have some limitations, such as small sample sizes and lack of randomization, which may affect the generalizability of the findings to humans. Further, research is needed to confirm these findings and optimize the laser parameters for clinical applications.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Low-level laser irradiation facilitates fibronectin and collagen type I turnover during tooth movement in rats. Lasers Med Sci. 2010;25:25-31.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Lasers Surg Med. 2000;26:282-91.
- [CrossRef] [Google Scholar]
- Low-level laser therapy increases interleukin-1β in gingival crevicular fluid and enhances the rate of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2018;154:535-44.e5.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of micro-osteoperforations on the rate of tooth movement. Am J Orthod Dentofacial Orthop. 2013;144:639-48.
- [CrossRef] [PubMed] [Google Scholar]
- Activation of latent TGF-β1 by low-power laser in vitro correlates with increased TGF-β1 levels in laser-enhanced oral wound healing. Wound Repair Regen. 2007;15:866-74.
- [CrossRef] [PubMed] [Google Scholar]
- Low level laser therapy (LLLT) as an effective therapeutic modality for delayed wound healing. Ann N Y Acad Sci. 2005;1056:486-93.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of low-level laser (GaAl-As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol. 2006;101:283-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed Laser Surg. 2006;24:705-14.
- [CrossRef] [PubMed] [Google Scholar]
- Low-level laser effects on simulated orthodontic tension side periodontal ligament cells. Photomed Laser Surg. 2013;31:72-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of 660 and 780 nm low-level laser therapy on neuromuscular recovery after crush injury in rat sciatic nerve. Lasers Surg Med. 2010;42:673-82.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin 1 beta, interleukin 6, beta 2-microglobulin, and transforming growth factor-α in gingival crevicular fluid from human periodontal disease. Arch Oral Biol. 1999;44:535-9.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukins 2, 6, and 8 levels in human gingival sulcus during orthodontic treatment. Am J Orthod Dentofacial Orthop. 2006;130:7.e1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of continuous and interrupted orthodontic force on interleukin-1β and prostaglandin E2 production in gingival crevicular fluid. Am J Orthod Dentofacial Orthop. 2004;125:168-77.
- [CrossRef] [PubMed] [Google Scholar]
- Composition changes in gingival crevicular fluid during orthodontic tooth movement: Comparisons between tension and compression sides. Eur J Oral Sci. 2006;114:416-22.
- [CrossRef] [PubMed] [Google Scholar]
- Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. Am J Orthod Dentofacial Orthop. 2001;119:307-12.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative evaluation of interleukin 1 beta and prostaglandin E2 with and without low-level laser therapy during en masse retraction. Contemp Clin Dent. 2018;9:267-75.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of diode laser (980 nm) on orthodontic tooth movement and interleukin 6 levels in gingival crevicular fluid in female subjects. Lasers Med Sci. 2016;31:1751-9.
- [CrossRef] [PubMed] [Google Scholar]
- Low-energy laser irradiation stimulates the tooth movement velocity via expression of M-CSF and c-fms. Orthod Waves. 2007;66:139-48.
- [CrossRef] [Google Scholar]
- Low-energy laser stimulates tooth movement velocity via expression of RANK and RANKL. Orthod Craniofacial Res. 2008;11:143-55.
- [CrossRef] [PubMed] [Google Scholar]
- Low-energy laser irradiation facilitates the velocity of tooth movement and the expressions of matrix metalloproteinase-9, cathepsin K, and alpha(v) beta(3) integrin in rats. Eur J Orthod. 2010;32:131-9.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of low-level laser therapy on tooth movement during canine distalization. Lasers Med Sci. 2017;32:757-64.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of low intensity laser therapy (LILT) on MMP-9 expression in gingival crevicular fluid and rate of orthodontic tooth movement in patients undergoing canine retraction: A randomized controlled trial. Int Orthod. 2020;18:330-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical research: Low-level laser therapy in accelerating orthodontic tooth movement. BMC Oral Health. 2021;21:324.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of low-intensity laser therapy in reducing treatment time and orthodontic pain: A clinical investigation. Am J Orthod Dentofacial Orthop. 2012;141:289-97.
- [CrossRef] [PubMed] [Google Scholar]
- Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofacial Orthop. 1997;111:525-32.
- [CrossRef] [PubMed] [Google Scholar]







