Translate this page into:
Age-related osteogenesis on lateral force application to rat incisor – Part II: Bony recession and cortical remodeling

*Corresponding author: Kee-Joon Lee, Department of Orthodontics, College of Dentistry, Institute of Craniofacial Deformity, Yonsei University, Seoul, Korea. orthojn@yuhs.ac
-
Received: ,
Accepted: ,
How to cite this article: Kim J, Baik H, Mo S, Giap H, Lee K. Age-related osteogenesis on lateral force application to rat incisor – Part II: Bony recession and cortical remodeling. APOS Trends Orthod 2021;11:174-82.
Abstract
Objective:
The aim of this study is to analyze the age-related changes in the bony recession and cortical bone remodeling induced by lateral orthodontic tooth movement, using a three-dimensional micro-computed tomography (CT) analysis.
Material and Methods:
A total of 40 male Sprague-Dawley rats were divided into two distinct age groups (young, 10 weeks and adult, 52 weeks). Double-helical springs exerting 40 g of force were applied to central incisors to analysis of changes in lateral cortical bone and tooth movement with age and time. The young and adult rats were divided into four subgroups, T0 (0 week), T1 (1 week), T2 (2 weeks), and T3 (3 weeks), depending on the period of wearing the appliance. Micro-CT image was taken on each dissected pre-maxilla specimen. In each subgroup, distance between the center of teeth, suture width, tooth displacement, bony recession, and bone volume was evaluated.
Results:
The changes in the distance between the center of teeth and the suture width were significantly greater in the young group. However, the change in the tooth displacement showed no significant difference between groups. In the young group, bony recession of outer cortical layer was observed at T1 (P < 0.05), but the amount of recession gradually decreased at T2 and T3. In contrast, in the adult group, bony recession increased gradually over observation period (P < 0.05). The bone volume decreased at T1 (P < 0.05), but recovered at T2 and T3 in both groups.
Conclusion:
The compensatory bone formation occurs in the pressure side of cortical bone more significantly in the young group than in the adult according to the lateral displacement of incisor in rats. The reduced bone reaction in the adult is considered a limiting factor of the excessive tooth movement in the compromised treatment of skeletal malocclusion.
Keywords
Recession
Age
Lateral movement
Cortical bone
INTRODUCTION
Tooth movement against surrounding cortical plate is often indicated for lateral expansion of constricted arch or retraction of anterior teeth following premolar extraction. The movement, however, may cause negative effects such as root resorption, bony recession, dehiscence, and fenestration.[1,2]
According to the pressure-tension theory, orthodontic forces are known to cause changes in the blood flow and to alter the cell behaviors, leading to bone resorption on the pressure side and bone apposition on the tension side bone adjacent to the periodontal ligament. In addition, active bone formation on the periosteal side is necessary for maintaining the tooth support by the alveolar housing, which was named as “compensatory bone formation.”[3]
Ashizawa and Sahara[4] and Verna et al.[5] reported new alveolar bone formation in the periosteal area on the pressure side during orthodontic tooth movement. Frost[6] supported the idea that orthodontic force is recognized as a stimulus, increases biologic activity, and promotes bone remodeling. In contrast, Garib et al.[7] demonstrated an average of 7.1 mm bone dehiscence in the first premolars and 3.8 mm in the first molars in growing patients following rapid maxillary expansion. Moreover, other studies described negative effects such as gingival and/or alveolar bone recession related to the labiolingual movement of lower incisors.[8,9] Although it has been demonstrated that spontaneous bone reformation took place when the once proclined tooth was moved back to its original position,[10] most clinicians’ concern may be the bone response with time around the tooth at its relocated position.
The age has been considered as one of the determinants of bone remodeling associated with orthodontic tooth movement. The previous studies reported that the bone remodeling with tooth movement was slower in adults compared to that in growing patients[11,12] claiming that active bone-forming capacity, bone response to some stimulus, and the periodontal reaction under orthodontic force decrease with age. Bridges et al.[13] attributed faster tooth movement in younger groups than in old groups to the differences in density of alveolar bone.
Some studies, however, maintain conflicting reports. Kabasawa et al.[14] demonstrated that the effect of age on osteoblast/osteoclast activities under orthodontic stimulus was neglectable along a short-term observation of 7 days. King et al.[15] reported that the difference in bone remodeling with age was insignificant for the declined bone cell capacity in specific sites with age may be compensated by the number of cell.
In view of the various reports on bone response according to orthodontic tooth movement, an empirical three-dimensional in vivo observation of cortical bone recession under constant orthodontic force might be of help to understand the possible risks of orthodontic tooth movement against cortical plate. The purpose of this study was to examine the morphological changes in the lateral cortical plate in response to lateral tooth movement in young and old age groups, using a three-dimensional micro-CT analysis.
MATERIAL AND METHODS
Subjects
A total of 40 male Sprague-Dawley rats were divided into two distinct age groups (young, 10 weeks, 358.2 g body weight and adult, 52 weeks, 607.5 g body weight). The rats were housed in stainless steel cages in an air-conditioned environment and subjected to standard 12 h light, dark cycle. They were fed with a pellet diet and tap water and checked every day in regard to their health status. The rats in each group were evenly assigned to control, 1, 2, and 3 weeks’ group according to the experimental period.
This study was approved in advance by the Institutional Animal Care and Use Committee.
Appliance setting
After the intraperitoneal injection of the Rompun (Rompun, Bayer, Korea) and Zoletil (Zoletil50, Virbac Lab Carros, France) in the experiment group, holes were made on both central incisors using high-speed 1/4 round bur. The double-helical springs of 0.014” stainless steel wire were set in the holes and wires were bent to surround teeth to prevent the appliance from detachment. The helical springs exerted 40 g of force enough to displace central incisors laterally without additional activation according to Part I of this serial study.[16] No soft-tissue injury from the springs was observed during the experiment. The retained force was verified by remaining elasticity in springs after the sacrifice of rats [Figure 1].

- Appliance setting. (a) Holes made on rat incisors; (b) lateral movement of rat incisors after double-helical spring activation.
Reconstruction of three-dimensional image using microcomputed tomography (micro-CT)
Micro-CT was taken (Skyscan micro CT 1076, Skyscan, Kontich, Belgium) on each pre-maxilla area of rats which were dissected. The images were established into three-dimensional images using Rapidform 2006 (Inus Technology Inc., Seoul, Korea).
Landmarks and reference planes
The landmarks and reference planes were required for the quantitative analysis of changes in lateral cortical bone and tooth movement with age and time. They based on the measurement method used in previous animal experiments [Figures 2 and 3].[17]
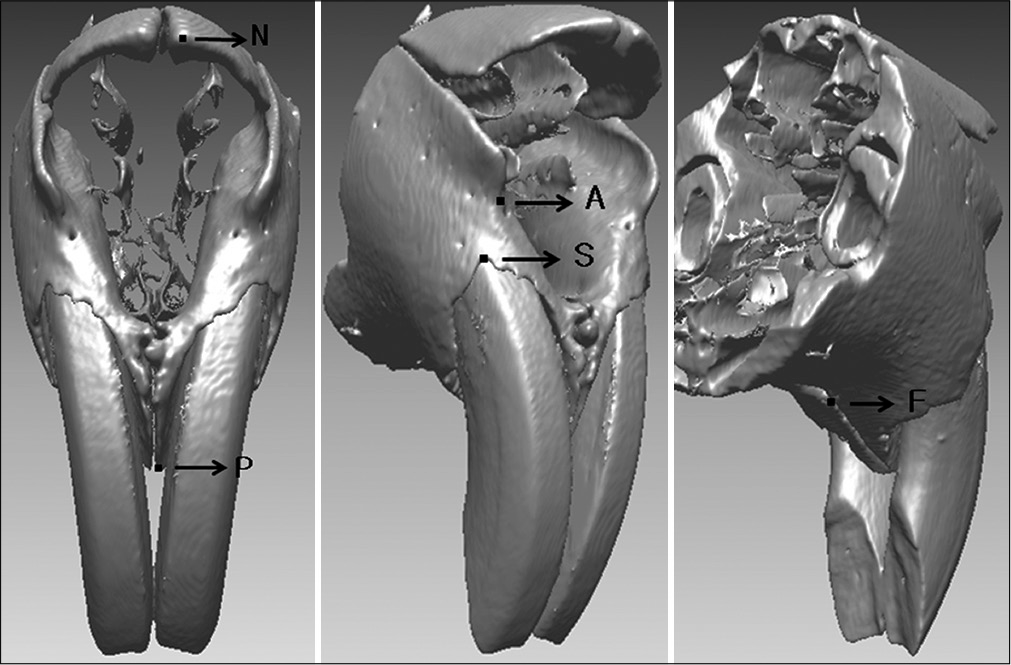
- Landmarks used in this study. N, internasal point; P, prosthion point; A, A point; S, incisive superior alveolar point; F, foramen incisivum anterior point.
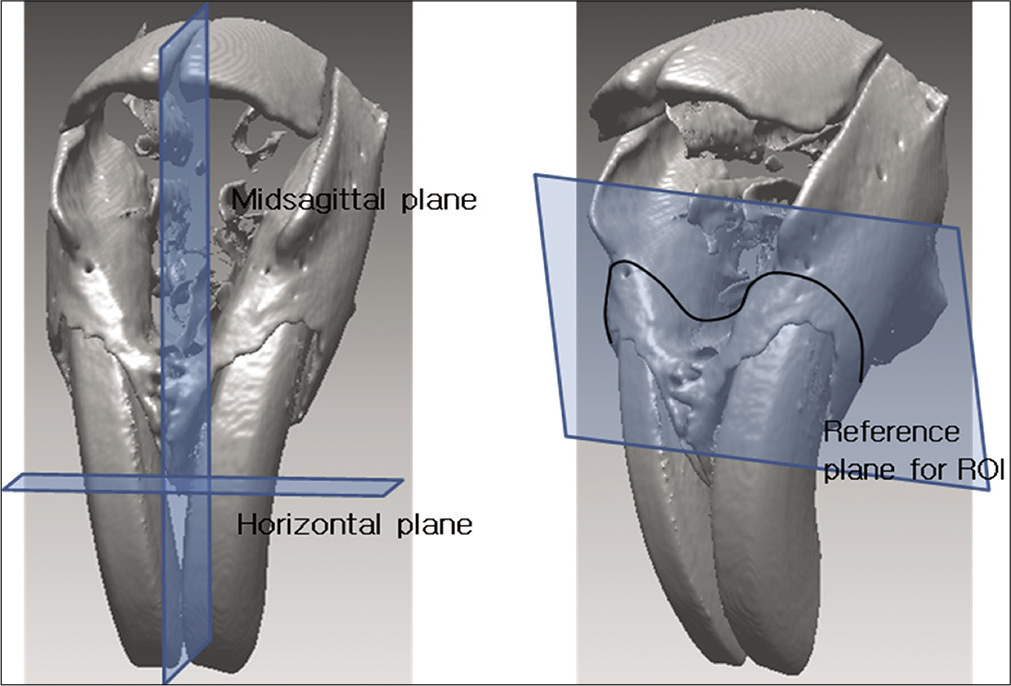
- Reference planes and region of interest.
Landmarks
Internasal point (N): Most anterior point of the internasal suture in the midsagittal plane
Prosthion point (P): Most inferior point on the alveolar bone between the upper two incisors
A point (A): Most concave point on anterior border of maxilla
Incisive superior alveolar point (S): Most superior edge point on the vestibular marginal alveolar bone of the upper central incisor
Foramen incisivum anterior point (F): Most anterior point of foramen incisivum.
Reference planes
Midsagittal reference plane: Plane that connects the midpoint of internasal point, prosthion point, and A point
Horizontal reference plane: Plane which is perpendicular to the midsagittal and includes prosthion points on left and right
Reference plane for region of interest (ROI): Plane that contains A points on left and right and the midpoint of foramen incisivum anterior point. (ROI refers to the underlying bone of reference planes.)
Measurement
The young and adult rats were divided into four subgroups, T0 (0 week), T1 (1 week), T2 (2 weeks), and T3 (3 weeks), depending on the experimental period. In each group, the following measurements were conducted from micro-CT images [Figures 4 and 5].
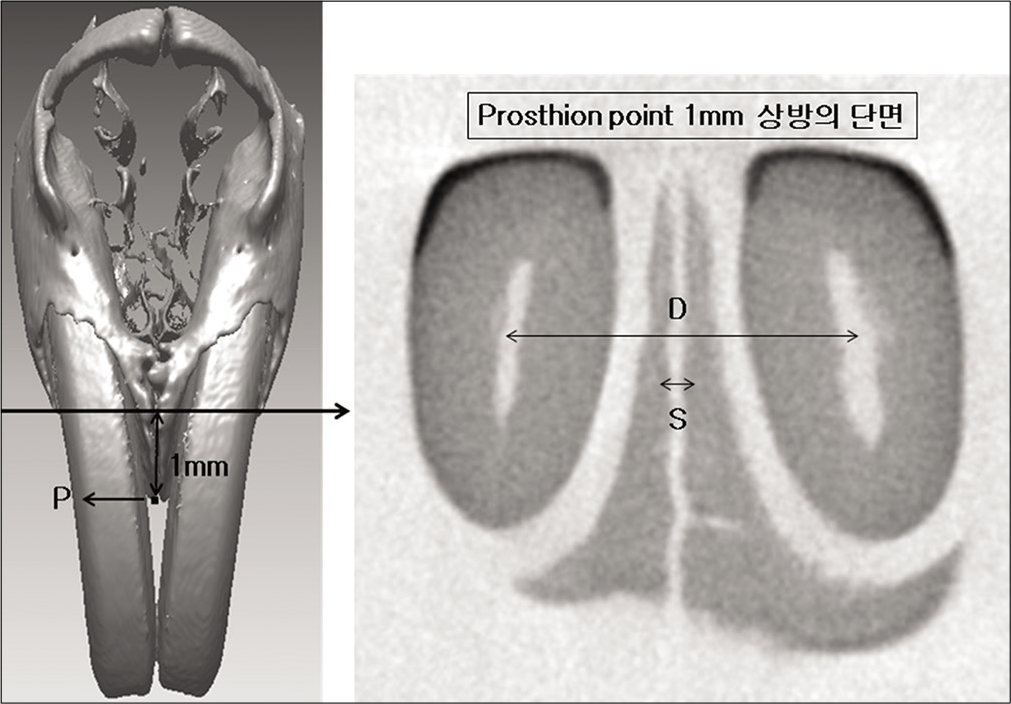
- Measurements for tooth movement. D, distance between the center of teeth; S, suture width.
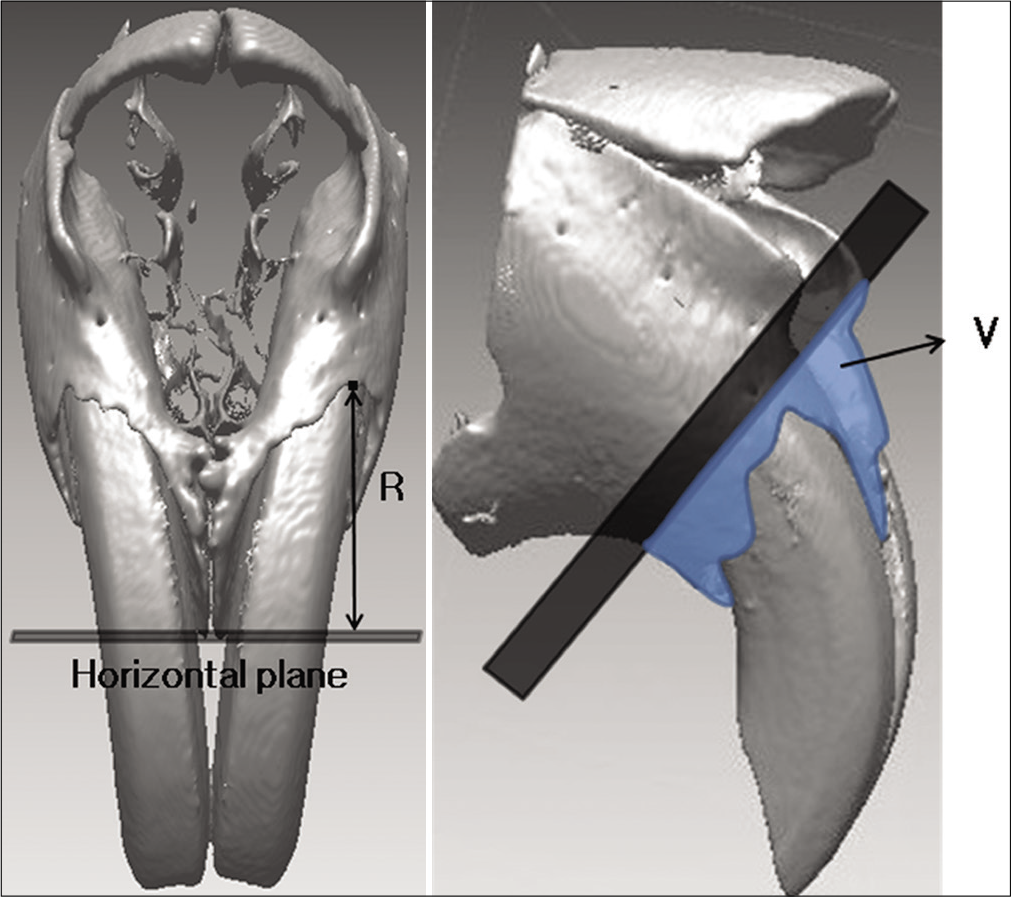
- Measurements for alveolar bone change. R, the perpendicular distance of incisive superior alveolar point to the horizontal plane; V, the volume of bone below the reference plane for ROI.
Distance between the center of teeth (D): Distance between the midpoint of central incisors at the section 1 mm above the prosthion point
Suture width (S): Distance between medial alveolar bone of central incisors at the section 1 mm above the prosthion point
Tooth displacement (M): Distance between the center of teeth (D) – suture width (S)
Bony recession (R): Perpendicular distance from the horizontal plane to the incisive superior alveolar point
Bone volume (V): Bone volume around central incisors below the reference plane for ROI
Statistical analysis
The measurements were statistically analyzed using SAS 9.1 Version (SAS Inc., North Carolina, USA).
Average and standard deviation of the five variables, distance between the center of teeth, suture width, tooth displacement, bony recession, and bone volume, were calculated
Turkey test as a post hoc test after ANOVA test was used in each age group for the comparison of different periods
Independent t-test was used in each period groups for the comparison of different ages
Spearman’s rank correlation analysis was used for correlation of five variables with age and time.
RESULTS
Analysis on the tooth movement in each group
The distance between the center of teeth, the suture width, and the tooth displacement commonly increased with time in both young and adult groups. The suture width showed no significant difference in both groups during the 1st week but gradually increased in both groups during at the 2nd week (P < 0.05) and the 3rd week (P < 0.001 in the young and P < 0.05 in the adult group), respectively. The tooth displacement increased significantly in both groups displaying P < 0.05, P < 0.01, and P < 0.001 in the 1st, 2nd, and 3rd week, respectively [Table 1; Figures 6 and 7].
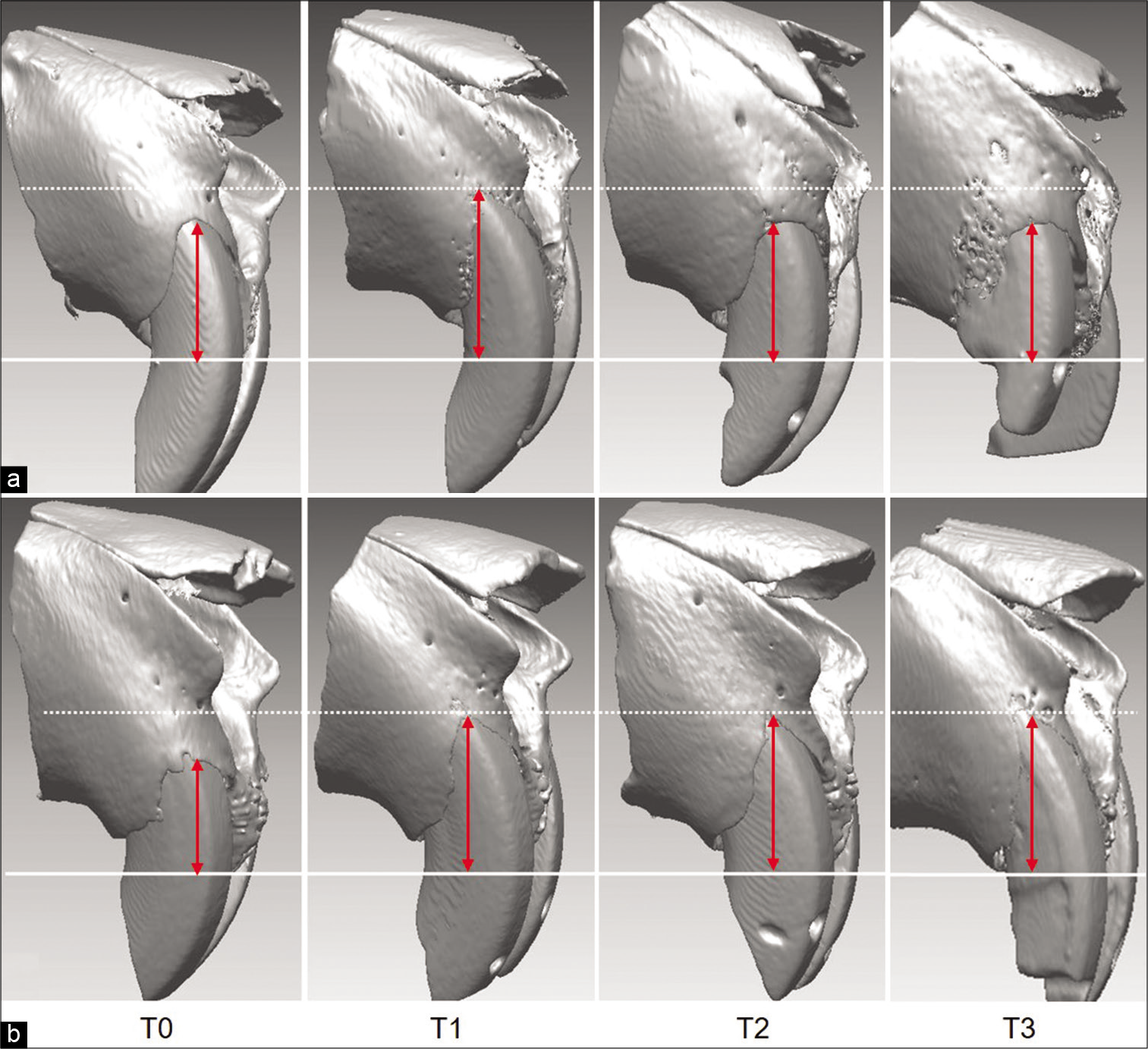
- Alveolar bone changes in the (a) young and (b) adult groups at T0 (0 week), T1 (1 week), T2 (2 weeks), and T3 (3 weeks).
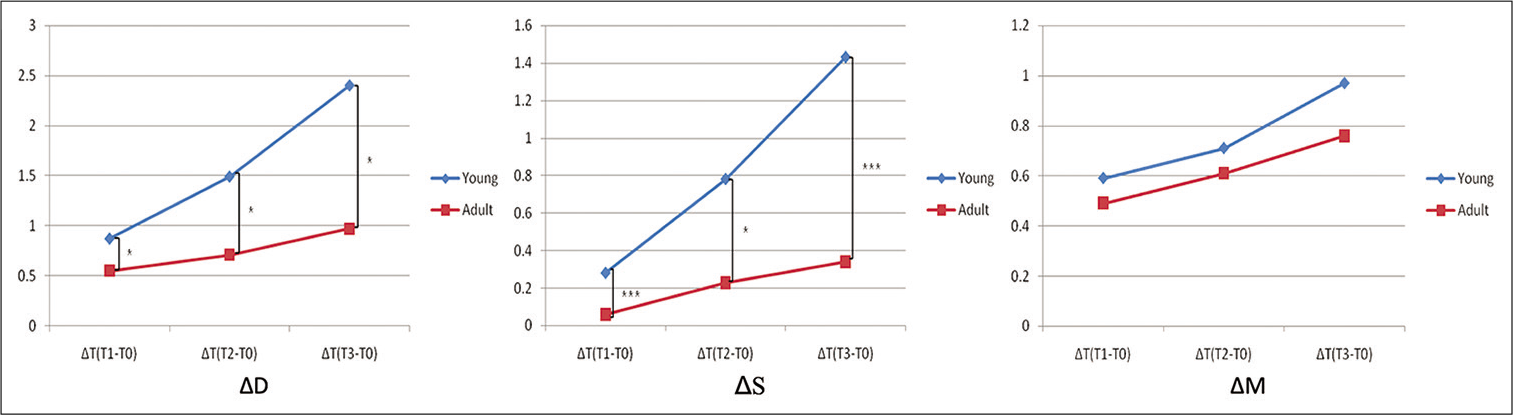
- The changes in the distance between the center of teeth (△D); the suture width (△S); and tooth displacement (△M) in the young (blue line) and adult group (red line).
| Time | Variables | Young group | Adult group | Sig. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | Sig. | Mean | S.D. | Sig. | ||||
| ΔT (T1-T0) | D | 0.87 | 0.12 | ** | 0.55 | 0.26 | * | * | |
| S | 0.28 | 0.06 | NS | 0.06 | 0.04 | NS | *** | ||
| M(D-S) | 0.59 | 0.07 | * | 0.49 | 0.24 | * | NS | ||
| ΔT (T2-T0) | D | 1.49 | 0.24 | *** | 0.84 | 0.17 | ** | * | |
| S | 0.78 | 0.33 | * | 0.23 | 0.03 | * | * | ||
| M(D-S) | 0.71 | 0.18 | ** | 0.61 | 0.17 | ** | NS | ||
| ΔT (T3-T0) | D | 2.40 | 0.38 | *** | 1.10 | 0.36 | *** | * | |
| S | 1.43 | 0.68 | *** | 0.34 | 0.21 | ** | *** | ||
| M(D-S) | 0.97 | 0.44 | *** | 0.76 | 0.20 | *** | NS |
The young group showed significantly larger value than the adult in the distance between the center of teeth (P < 0.05) and the suture width (P < 0.001) in all time point [Table 1; Figure 7]. The tooth displacement within the alveolar bone, however, showed no significant difference between the groups [Table 1; Figure 7].
Analysis on the alveolar bone recession in each group
An average of 0.82 mm (P < 0.05) bone recession in the lateral cortical bone was shown in the 1st week of the young group, however, the extent gradually decreased to 0.61 mm in the second and 0.18 mm in the 3rd week [Table 2; Figures 6 and 8]. While bone volume significantly decreased to 4.92 mm3 (P < 0.001) in the 1st week, only 0.84 mm3 reduction in the 2nd week, and 0.12 mm3 in the 3rd week were noted, implying no significant difference compared to the control group [Table 2; Figures 6 and 8].
| Time | Variables | Young group | Adult group | Sig. | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | Sig. | Mean | S.D. | Sig. | |||
| ΔT (T1-T0) | R (mm) | 0.82 | 0.44 | * | 1.04 | 0.44 | * | NS |
| V (mm3) | –4.92 | 2.02 | *** | –5.08 | 3.61 | * | NS | |
| ΔT (T2-T0) | R (mm) | 0.61 | 0.86 | NS | 1.50 | 0.50 | *** | NS |
| V (mm3) | –0.84 | 1.67 | NS | –4.00 | 1.73 | NS | * | |
| ΔT (T3-T0) | R (mm) | 0.18 | 0.70 | NS | 1.54 | 0.44 | *** | ** |
| V (mm3) | –0.12 | 1.26 | NS | –2.72 | 1.42 | NS | * | |
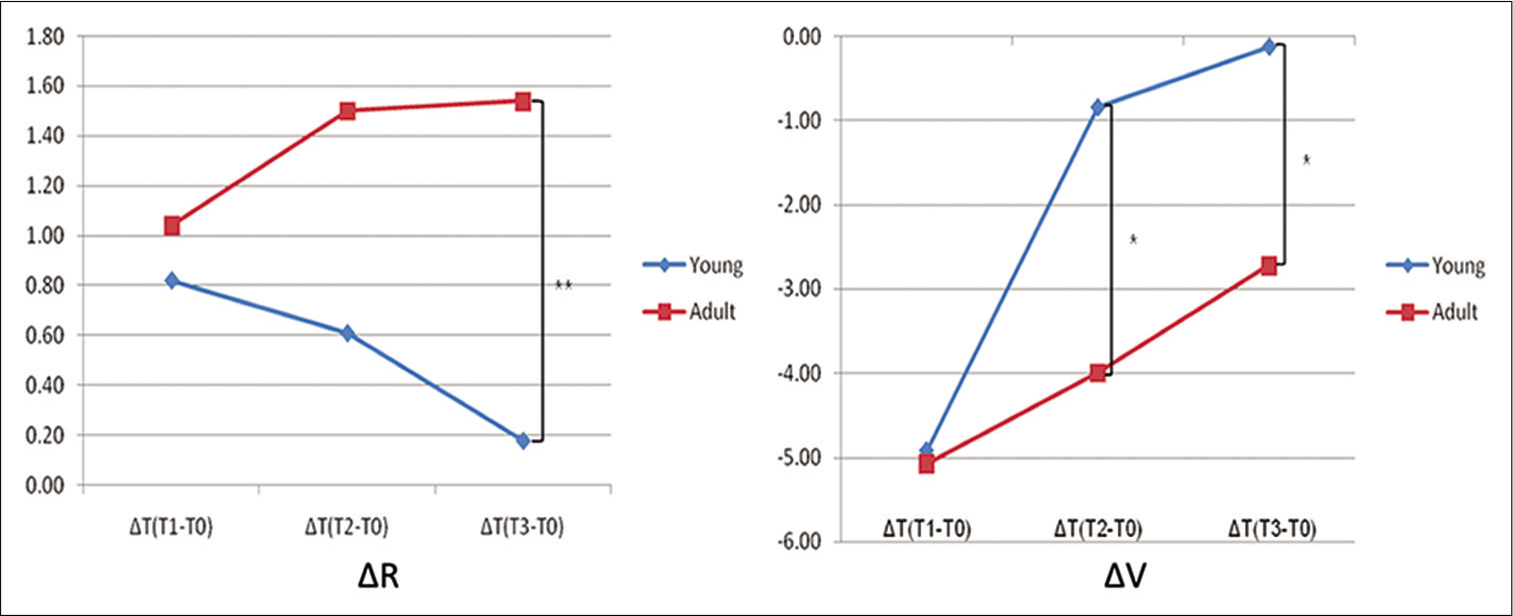
- Bony recession (ΔR) and bone volume (ΔV) change in the young (blue line) and adult group (red line).
In the adult group, the amount of bony recession of the lateral cortical bone showed an average of 1.04 mm (P < 0.05) in the 1st week and gradually increased to 1.50 mm (P < 0.001) in the 2nd week, and 1.54 mm (P < 0.001) in the 3rd week [Table 2; Figures 6 and 8]. The bone volume decreased an average of 5.08 mm3 (P < 0.05) in the 1st week, 4.00 mm3 in the 2nd week, and 2.72 mm3 in the 3rd week [Table 2; Figures 6 and 8].
In terms of group comparison, the bone recession and the decrease of bone volume in the young group were not significantly different from those in adult group at the 1st week. At the 2nd week, the amount of bone recession remained insignificant, while the bone volume recovered significantly in the young group (P < 0.05). At the 3rd week, the amount of bone recession and the decrease of bone volume were significantly smaller in the young group than in the adult [Table 2; Figures 6 and 8].
Correlation of each variable with age and time
At the 2nd week, the young (P < 0.001) and the adult (P < 0.01) groups showed significant correlation between the distance between the center of teeth and the bone volume. However, there was no significant correlation between the bone recession and the other variables in any group at any time [Tables 3 and 4].
| Young (T2) | D | S | M | R | V |
|---|---|---|---|---|---|
| D | 1.000 | ||||
| S | 0.700 | 1.000 | |||
| 0.188 | |||||
| M | 0.051 | –0.667 | 1.000 | ||
| 0.935 | 0.219 | ||||
| R | 0.100 | 0.100 | –0.205 | 1.000 | |
| 0.873 | 0.873 | 0.741 | |||
| V | 1.000 | 0.700 | 0.051 | 0.100 | 1.000 |
| <0.0001(***) | 0.188 | 0.935 | 0.873 |
| Adult (T2) | D | S | M | R | V |
|---|---|---|---|---|---|
| D | 1.000 | ||||
| S | 0.000 | 1.000 | |||
| 1.000 | |||||
| M | 0.821 | –0.564 | 1.000 | ||
| 0.089 | 0.322 | ||||
| R | 0.154 | –0.359 | 0.300 | 1.000 | |
| 0.805 | 0.553 | 0.624 | |||
| V | 0.975 | –0.154 | 0.900 | 0.100 | 1.000 |
| 0.005 (**) | 0.805 | 0.037 | 0.873 |
DISCUSSION
With increasing demands for the orthodontic treatment in adults and possibly increased range of tooth movement by the introduction of new technologies including temporary anchorage devices, the periosteal bone responses to tooth movement have also been brought into attention. The three-dimensional micro-CT recruited in this study was expected to better demonstrate the actual changes in bone contour at any given axial sections and enable the volumetric measurements, in contrast to the conventional two-dimensional radiographs. The allocation of age groups was described previously.[18,19] Despite the differences in the periodontal structures between murine and human, such as the relatively higher density of alveolar bone and the distinctive periodontal ligament fiber arrangement, thin lateral cortical plate covering the incisors was considered appropriate for the induction of bony recession, resembling buccal/labial cortical plate in human canine or premolar area. Only male rats were used in the experiment to eliminate the potential hormonal influence in females.[20]
The 40 g of orthodontic force was applied according to the previous studies, to induce noticeable biologic response.[21-23]
In spite of the lack of reactivation, the average retained force by the spring after the experimental period exceeded 50% of initial forces (data not shown), indicating that the force application was relatively constant. The pulpal damage, anticipated in the process of appliance setting, might be negligible referring to the studies stating that the effect of pulp vitality on tooth movement is insignificant.[24] The force might delay the eruption of rats’ incisors, which is possibly why all the appliances in this study were maintained and the amount of attrition was minimal through the 3 weeks of experiment.[25] However, consequent change in alveolar bone associated with the possible minute eruption might be the limitation of this study. Some of the incisors displayed slightly vertical displacement as well as lateral, which might have affected the periosteal changes. Therefore, all the measurements were averaged between right and left sides in each animal.
Since it was anticipated that the lateral force would induce both the suture separation and the tooth displacement, the two measurements had to be discriminated. Although greater overall tooth displacement in the young group compared to adults was found, the actual amount of tooth displacement within the alveolar socket was not significantly different between groups showing gradual increase with time, mainly due to the greater suture separation in young group [Table 1; Figure 7]. The result suggested that the introduced force induced equivalent tooth displacement within the alveolar bone in each age group. In the young rats, low bone density allowing easier bone bending, sufficient periodontal space, and abundant undifferentiated mesenchymal cells reserved for osteoblasts and/or osteoclasts have been shown to be associated with faster tooth movement, than in the aged rats.[13,26,27] Extension of the observation period may affect the amount of tooth movement, but the velocity of tooth movement was not the major concern in this study.
The bony recession significantly increased in the young group during the 1st week (P < 0.05), but gradually decreased at the 2nd and 3rd week, restoring the original level [Table 2; Figures 6 and 8]. The previous studies mostly focused on the healing of recession through lingual reposition of the labially displaced tooth.[10,28] In contrast, evidence of active bone formation on the periosteal side according to the lingual movement of molars was also shown by Shimpo et al.[29] Consistent with this finding, King et al.[30] reported that the experimental group under orthodontic force displayed constant bone remodeling after the removal of appliance. Spontaneous restoration of the bony recession against the constant lateral tooth movement was a novel finding in this study and implicated the presence of compensatory bone formation. Sequential recession and reformation of cortical bone were also an interesting finding in this study, implying the induction of osteogenesis stimulated by orthodontic tooth movement. King et al.[31] also stated that the cells in intact periodontal ligament migrated to damage periodontium with the help of factors such as recombinant human bone morphogenetic protein-2, and that the migrated cells proliferated and induced cell recruitment regenerating bone and cementum. Further study on the cellular/molecular behavior may reveal the underlying relevant mechanisms.
In the adult group, however, the bone recession increased with time, presumably attributed by the differences in bone reaction with age [Table 2; Figures 6 and 8]. Jager et al.[11] stated that the decreased number of cells related to bone formation resulted in the decline bone remodeling ability. This study, however, the increment in bone recession per unit hour gradually decreased in the adult group. There was little change between the 2nd and 3rd week group with 1.50 mm and 1.54 mm, respectively. This result means that at least limited compensatory bone formation in the adult group may be present against the continuous lateral tooth movement.
The bone volume significantly decreased in the 1st week in both young (P < 0.001) and adult (P < 0.05) groups, but recovered afterward [Table 2; Figures 6 and 8]. The changes of bone volume during the lateral movement of incisors might be attributed to the bone formation at suture area, the inner, and the outer cortical bone areas. Hence, it is not possible to distinguish the exact influence of each part on the gross change in the bone volume. Melsen et al.[32] indicated that except for the excessive tooth movement over the limit of alveolar bone, the bone resorption and formation in the periodontal ligament area were in balance maintaining the total volume. Accordingly, the recovery of the bone volume in both groups is consistent to the sequential recession and restoration on the lateral cortical area, implying the presence of bone formation in a timely manner.
The changes in bone volume with age showed no significant difference between groups in the 1st week [Table 2; Figure 8]. However, the decrement of bone volume in the 2nd and 3rd week groups of young group was significantly smaller than that of the adult group (P < 0.05), indicating more active osteogenesis in any direction [Table 2; Figure 8]. The amount of bone recession had no significant correlation with other variables at any time. The bone recession seemed to be more related to individual bone formation ability rather than to tooth displacement.
Regarding the amount of tooth movement, Ren et al. stated that though initial tooth movement was more rapid in younger age, there was no difference between young and adult age groups after the 4 weeks of orthodontic force.[33] Relevant to this, actual tooth displacement in this study was not different depending on age, which justified the comparison of the bone volume between groups.
In summary, compensatory bone formation in the lateral cortical bone of the pressure side was quantitatively measured under the lateral force on incisors. Significantly greater bone formation in the young group was presumably due to the limited bone reaction in the adult group. Hence, excessive tooth movement beyond the alveolar housing may be recovered by subsequent bone formation in young age groups, which may encourage nonsurgical treatment in various skeletal discrepancies. Therefore, next series of this study would deal with comprehensive histologic analysis to reveal underlying cellular mechanism of the findings in this study.
CONCLUSION
The results verify that the compensatory bone formation occurs in the pressure side of cortical bone during the orthodontic tooth movement with more activity in the young group than in the adult. The reduced bone reaction in the adult is considered the limiting factor of the excessive tooth movement in the compromised treatment of skeletal malocclusion.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
This study was supported by a faculty research grant of Yonsei University College of Dentistry, Grant/Award Number: 2017-0012.
Conflicts of interest
There are no conflicts of interest.
References
- Correlation between cortical plate proximity and apical root resorption. Am J Orthod Dentofacial Orthop. 1998;114:311-8.
- [CrossRef] [Google Scholar]
- Faciolingual tooth movement: Its influence on the root and cortical plate. Am J Orthod. 1973;64:278-302.
- [CrossRef] [Google Scholar]
- Correlation of bone resorption and formation with physical behavior of loaded bone. J Dent Res. 1965;44:33-41.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative evaluation of newly formed bone in the alveolar wall surrounding the root during the initial stage of experimental tooth movement in the rat. Arch Oral Biol. 1998;43:473-84.
- [CrossRef] [Google Scholar]
- Histomorphometric study of bone reactions during orthodontic tooth movement in rats. Bone. 1999;24:371-9.
- [CrossRef] [Google Scholar]
- The utah paradigm of skeletal physiology: An overview of its insights for bone, cartilage and collagenous tissue organs. J Bone Miner Metab. 2000;18:305-16.
- [CrossRef] [PubMed] [Google Scholar]
- Periodontal effects of rapid maxillary expansion with tooth-tissue-borne and tooth-borne expanders: A computed tomography evaluation. Am J Orthod Dentofacial Orthop. 2006;129:749-58.
- [CrossRef] [PubMed] [Google Scholar]
- Periodontal status of mandibular incisors following excessive proclination. A study in adults with surgically treated mandibular prognathism. Am J Orthod Dentofacial Orthop. 1987;91:225-32.
- [CrossRef] [Google Scholar]
- Changes in alveolar bone thickness due to retraction of anterior teeth. Am J Orthod Dentofacial Orthop. 2002;122:15-26.
- [CrossRef] [PubMed] [Google Scholar]
- Bone regeneration in alveolar bone dehiscences related to orthodontic tooth movements. Eur J Orthod. 1983;5:105-14.
- [CrossRef] [PubMed] [Google Scholar]
- Histomorphometric study of age-related changes in remodelling activity of human desmodontal bone. J Anat. 1996;189:257-64.
- [Google Scholar]
- Influences of aging changes in proliferative rate of PDL cells during experimental tooth movement in rats. Angle Orthod. 1997;67:67-72.
- [Google Scholar]
- The effect of age on tooth movement and mineral density in the alveolar tissues of the rat. Am J Orthod Dentofacial Orthop. 1988;93:245-50.
- [CrossRef] [Google Scholar]
- Effect of age on physiologic and mechanically stressed rat alveolar bone: A cytologic and histochemical study. Int J Adult Orthodon Orthognath Surg. 1996;11:313-27.
- [Google Scholar]
- Histomorphometric study of alveolar bone turnover in orthodontic tooth movement. Bone. 1991;12:401-9.
- [CrossRef] [Google Scholar]
- Age-related osteogenesis on lateral force application to rat incisor-Part I: Premaxilla suture remodeling. APOS Trends Orthod. 2020;10:38-45.
- [CrossRef] [Google Scholar]
- The influence of the masticatory hypofunction on the craniofacial growth and development in rats. Am J Orthod Dentofacial Orthop. 1997;111:189-98.
- [CrossRef] [Google Scholar]
- The rat as a model for orthodontic tooth movement-a critical review and a proposed solution. Eur J Orthod. 2004;26:483-90.
- [CrossRef] [PubMed] [Google Scholar]
- Comparing rat's to human's age: How old is my rat in people years? Nutrition. 2005;21:775-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of nitric oxide in orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 2004;126:608-14.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of orthodontic force on periodontal tissue metabolism. A histologic and biochemical study in normal and hypocalcemic young rats. Am J Orthod Dentofacial Orthop. 1988;93:486-95.
- [CrossRef] [Google Scholar]
- C-fos expression in rat brain nuclei following incisor tooth movement. J Dent Res. 2004;83:50-4.
- [CrossRef] [PubMed] [Google Scholar]
- Force magnitude effects upon osteoprogenitor cells during premaxillary expansion in rats. Angle Orthod. 1992;62:197-202.
- [Google Scholar]
- Endodontic-orthodontic relationshops: A review of integrated treatment planning challenges. Int Endod J. 1999;32:343-60.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of load duration on long-term recovery of the eruptive function in the rat incisor. Am J Orthod Dentofacial Orthop. 1988;93:310-4.
- [CrossRef] [Google Scholar]
- Effect of aging on functional changes of periodontal tissue cells. Ann Periodontol. 1998;3:350-69.
- [CrossRef] [PubMed] [Google Scholar]
- Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech Ageing Dev. 2008;129:163-73.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of incisor repositioning on monkey periodontium after expansion through the cortical plate. Am J Orthod. 1982;82:23-32.
- [CrossRef] [Google Scholar]
- Regulatory mechanisms of periodontal regeneration. Microsc Res Tech. 2003;60:491-502.
- [CrossRef] [PubMed] [Google Scholar]
- Alveolar bone turnover and tooth movement in male rats after removal of orthodontic appliances. Am J Orthod Dentofacial Orthop. 1997;111:266-75.
- [CrossRef] [Google Scholar]
- Bone morphogenetic protein-2 stimulates cell recruitment and cementogenesis during early wound healing. J Clin Periodontol. 2001;28:465-75.
- [CrossRef] [PubMed] [Google Scholar]
- Current Controversies in Orthodontics Chicago: Quintessence Publishing Company; 1991.
- [Google Scholar]
- Age effect on orthodontic tooth movement in rats. J Dent Res. 2003;82:38-42.
- [CrossRef] [PubMed] [Google Scholar]







