Translate this page into:
An In vitro Assessment of Antibacterial Activity of Three Self-etching Primers Against Oral Microflora
Address for correspondence: Dr. Sneha Dipak Shinde, PG Resident, Department of Orthodontics and Dentofacial Orthopedics, Maratha Mandal’s NGH Institute of Dental Sciences and Research Centre, Bauxite Road, Belagavi, Karnataka, India. E-mail: snehpratik82@gmail.com
This article was originally published by Wolters Kluwer and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Aims
This study aims to evaluate and compare the antibacterial activity of three self-etching primers (SEP), namely, Transbond plus, Reliance, and Gluma against commonly encountered oral microflora (Streptococcus mutans, Lactobacillus acidophilus, and Actinomyces viscosus).
Subjects and Methods
The antibacterial activity of the three SEPs was examined against microorganisms using agar diffusion test (ADT) and minimum inhibitory concentration (MIC). In ADT, Whatman’s filter paper disc of 5 mm was loaded with primer and polymerized. This was placed on previously inoculated brain heart infusion and blood agar plates and was incubated for 48– 72 h at 37°C according to the microorganism. For assessing MIC serial dilution method was used.
Statistical Analysis Used
Data were analyzed with Kruskal–Wallis (P < 0.001) and Mann–Whitney tests.
Results
Only Transbond plus SEP and Reliance SEP produced a clear growth inhibition halo against S. mutans, L. acidophilus and A. viscosus. Gluma SEP did not show any growth inhibition halo against S. mutans, L. acidophilus, and A. viscosus.
Conclusions
TSEP and Reliance SEP did show antibacterial activity in an in vitro environment. Therefore, this study concludes that the use of these SEPs may contribute to a reduction in bacterial colonization.
Keywords
Agar diffusion test
antibacterial activity
minimum inhibitory concentration
self-etching primers
Introduction
The orthodontic treatment involves placement of a fixed orthodontic appliance and is always recognized for its long treatment duration, which leads to increase in the level of Streptococcus mutans and Lactobacillus acidophilus in saliva and dental plaque.[1] The placement of fixed appliance also hampers the maintenance of oral hygiene. This leads to decalcification of enamel surface leading to caries formation. The further formation of plaque and calculus leads to gingival recession and periodontal diseases. S. mutans and Actinomyces viscosus bacterias are cariogenic in nature and also are associated with periodontal diseases, thus hampering the prognosis of orthodontic treatment.
Orthodontic resins which are used for bracket bonding on tooth enamel contribute to demineralization as it has rough surfaces, ideal for colonization by oral microorganisms. For this reason, rigorous elimination of resin remains, and optimum oral hygiene maintenance during orthodontic treatment are recommended.[2]
Conventional methods for bonding orthodontic brackets to enamel surface necessitates 3 different agents: an enamel conditioner, a primer solution, and an adhesive resin. Phosphoric acid solution is the most widely used enamel conditioner. The enamel conditioner is applied over the enamel surface for 15–30 s. At the end of etching period, the conditioner is rinsed off the teeth with abundant water spray. After the teeth are completely dry and frosty white, a thin layer of primer is painted over the etched enamel surface. After this, the operator proceeds with the bonding procedure.[3]
The introduction of new seventh generation acid etch primers has attracted considerable interest as they combine etching and priming into one, eliminating the need to rinse and thus avoiding damage to gingival tissue. The use of self-etching primers (SEPs) reduces clinical steps and saves clinical operation time and application requires simply drying with air.[3] Further, they minimize the amount of enamel lost during etching.
This step promotes an effective, long lasting seal of tooth structure to avoid gap formation at enamel-adhesive interface causing microfiltration. The residual bacteria count is high with SEPs as the smear layer is not washed away.[4] Therefore, adhesive systems that possess antibacterial activity may be useful for eliminating harmful effects caused by bacteria and will contribute to a better prognosis. With this objective, SEPs with supposedly antibacterial properties have been introduced into the market and are commonly available. The most commonly used are Transbond Plus SEP (3M Unitek, Monrovia, California, USA), a self-etching fluoride-releasing orthodontic adhesive. The other such SEPs are, Reliance SEP (Reliance Orthodontic Products, Itasca) and Gluma SEP (Heraeus Kulzer, Germany).
Several studies have attributed the antibacterial effect of SEPs to their low pH, but these studies have been inconclusive. Therefore, this study was taken up to evaluate the antibacterial activity of three commercially available SEPs against S. mutans, L. acidophilus and A. viscosus. Hence, the objective of the study was to evaluate and compare the antibacterial activity of SEPs (Transbond plus SEP, Reliance SEP, and Gluma SEP) against S. mutans, L. acidophilus, and A. viscosus.
Subjects and Methods
Source of data was the microbial strains. The test microorganisms used in the study:
S. mutans (ATCC no. 25175)
L. acidophilus (ATCC no. 4356)
A. viscosus (ATCC no. 15987).
Preparation of specimen
Autoclaved Whatman’s filter paper discs of diameter 5 mm were impregnated with 20 µl of each test sample (i.e., SEP) and polymerized for 20 s under strict sterile conditions in laminar air flow.
Agar diffusion test
Individual petri plates with brain heart infusion (BHI) agar for S. mutans and blood agar for L. acidophilus and A. viscosus were inoculated. In each petri plate five discs, three SEPs and two controls were placed with sterile forceps. Plates were then placed in the incubator according to respective test organism as follows: S. mutans - 37°C for 24–48 h, L. acidophilus - 35°C for 48–72 h, and A. viscosus - 35°C for 48–72 h.
After the appropriate incubation period, the diameter of the growth inhibition halo was measured in mm with vernier caliper [Figures 1-3]. The three SEPs and two controls used in the study to check the antibacterial activity were marked as groups:

- Agar diffusion test to detect antibacterial activity of self-etching primers against Streptococcus mutans
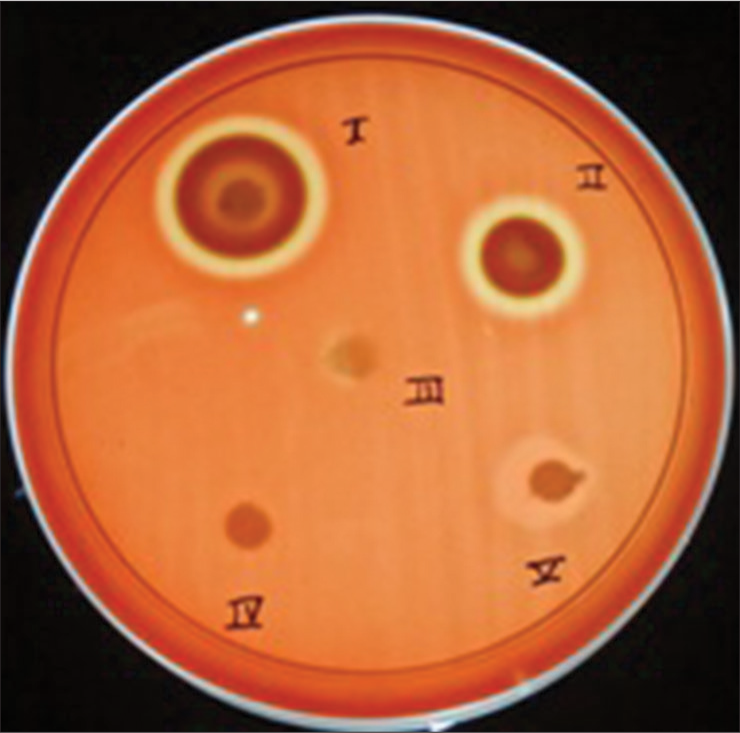
- Agar diffusion test to detect antibacterial activity of self-etching primers against Lactobacillus acidophilus

- Agar diffusion test to detect antibacterial activity of self-etching primers against Actinomyces viscosus
Group I - Transbond plus SEP
Group II - Reliance SEP
Group III - Gluma SEP
Group IV - Normal saline (negative control)
Group V - Acetone (positive control).
The three SEPs used as test specimen for a particular organism were tested in a triplet form.
Minimum inhibitory concentration
Procedure:
Nine dilutions of each primer were done with BHI and Thioglycollate broth for minimum inhibitory concentration (MIC)
In the initial tube (first tube), 100 µl of primer was added into the 300 µl of BHI broth for S. mutans and Thioglycollate broth for L. acidophilus and A. viscosus
Then from the initial tube, 200 µl was transferred to the first tube containing 200 µl of broth. This was considered as 10−1 dilution
From 10−1 diluted tube, 200 µl was transferred to the second tube to make 10−2 dilution
The serial dilution was repeated up to 10−9 dilution for each test sample [Figure 4]
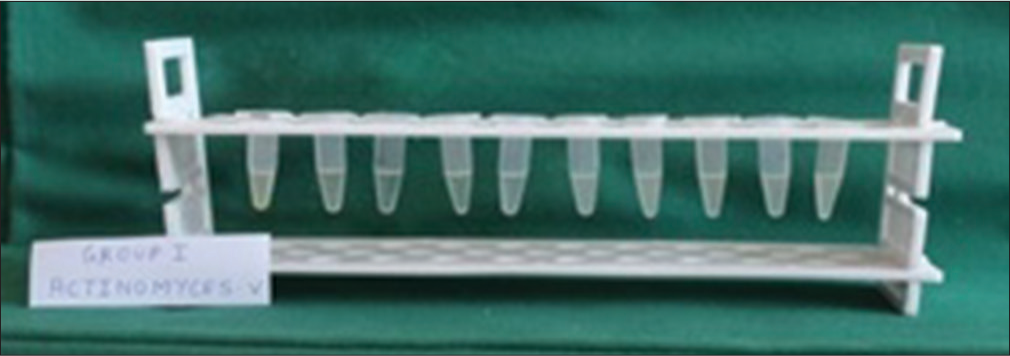 Figure 4
Figure 4- Minimum inhibitory concentration of Transbond plus self-etching primer against Actinomyces viscosus
The concentrations of the SEPs achieved by this serial dilution method were as following 100, 50, 25, 12.5, 6.25, 3.12, 1.6, 0.8, 0.4, 0.2 µg/ml
In each serially diluted tube, 200 µl of maintained stock culture suspension of required organism was added
The tubes were incubated for 24 h for S. mutans and 48–72 h for L. acidophilus and A. viscosus
After the incubation period, the MIC value was determined by visualizing each series of tubes, and the last tube with clear supernatant was taken as the MIC value.
The clear supernatant was considered to be without any growth. Turbidity in the MIC tube indicated the growth of the bacteria implying that the bacteria were resistant to the SEPs. All the sample dilutions were compared qualitatively by observing turbidity to assess the antibacterial activity.
Statistical analysis
The inhibition halos data produced by the different primers on each bacterial strain was evaluated using Kruskal– Wallis ANOVA test. Mann–Whitney U-test was used to find a significant difference for two independent samples. P < 0.001 was considered as statistically significant. Data were subjected to statistical analysis using SPSS 10.0 for social sciences (SPSS 10.0 Inc. IBM, Chicago, USA). Data of MIC were assessed qualitatively based on their turbidity.
Results
Agar diffusion test
The results of growth inhibition halos, using agar diffusion test (ADT) were obtained. The readings obtained are presented as follows [Table 1].
| Group | N | Mean | Std. Deviation | Range | Percentiles | |||
|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | 25th | 50th (Median) | 75th | ||||
| Group 1 | ||||||||
| S.mutans | 9 | 11.44 | 1.13 | 10.0 | 13.0 | 10.5 | 11.0 | 12.5 |
| L.acidophilus | 9 | 15.56 | 2.74 | 12.0 | 20.0 | 12.5 | 16.0 | 17.5 |
| A.viscosus | 9 | 16.33 | 1.41 | 14.0 | 18.0 | 15.0 | 17.0 | 17.5 |
| Group 2 | ||||||||
| S.mutans | 9 | 9.22 | 0.97 | 8.0 | 11.0 | 8.5 | 9.0 | 10.0 |
| L.acidophilus | 9 | 11.22 | 2.05 | 8.0 | 14.0 | 9.0 | 12.0 | 12.5 |
| A.viscosus | 9 | 19.33 | 1.73 | 17.0 | 22.0 | 18.0 | 19.0 | 21.0 |
| Group 3 | ||||||||
| S.mutans | 9 | 0.00 | 0.00 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| L.acidophilus | 9 | 0.00 | 0.00 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| A.viscosus | 9 | 0.00 | 0.00 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Group 4 | ||||||||
| S.mutans | 9 | 0.00 | 0.00 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| L.acidophilus | 9 | 0.00 | 0.00 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| A.viscosus | 9 | 0.00 | 0.00 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Group 5 | ||||||||
| S.mutans | 9 | 12.11 | 1.27 | 10.0 | 14.0 | 11.0 | 12.0 | 13.0 |
| L.acidophilus | 9 | 10.89 | 0.33 | 10.0 | 11.0 | 11.0 | 11.0 | 11.0 |
| A.viscosus | 9 | 12.67 | 0.50 | 12.0 | 13.0 | 12.0 | 13.0 | 13.0 |
In general, for all bacterial strains, Transbond plus SEP and Reliance SEP showed growth inhibition halos data statistically significant. Transbond plus SEP showed significantly greater growth inhibition halos against S. mutans [Table 1] and L. acidophilus [Table 1] than Reliance SEP (P < 0.001) and Gluma SEP (P < 0.001). Reliance SEP showed significantly greater growth inhibition halos against A. viscosus [Table 1] than Transbond plus SEP (P < 0.001) as compared to Gluma SEP.
Thus, against S. mutans and L. acidophilus, antibacterial activity of Transbond plus SEP was greater than Reliance SEP. Against A. viscosus antibacterial activity of Reliance SEP was greater than Transbond plus SEP. Acetone (positive control) in general showed significant antibacterial activity against all three bacterial strains. Neither saline nor Gluma SEP showed antibacterial activity against either of the bacterial strains [Table 1]. When inhibition halos produced by each SEP were evaluated comparing three bacterial strains, significant difference was found between those produced by Transbond plus SEP and Reliance SEP against all three bacterial strains (P < 0.001). The comparison of antibacterial activity of self-etching primers (SEP) tested against microorganisms can be seen [Graph 1].
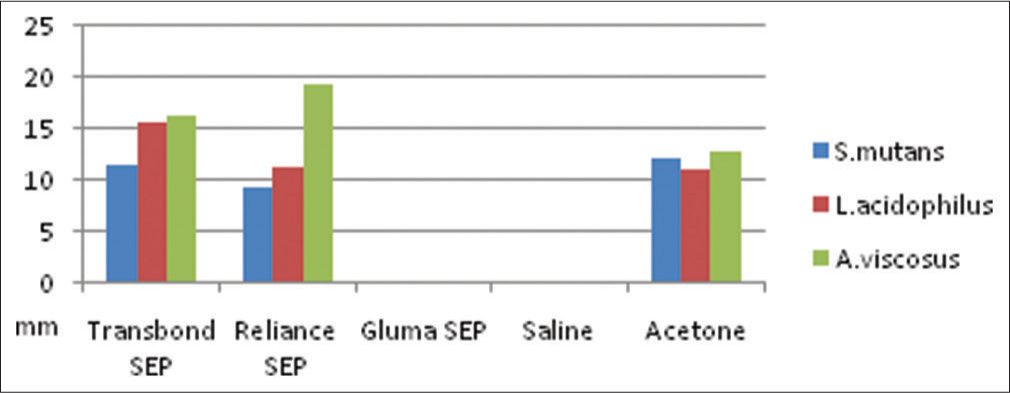
- Comparison of antibacterial activity of self-etching primers tested against each bacterial strain
Minimum inhibitory concentration
Qualitative assessment of the MIC results was made by observing the turbidity. The groups tested showed sensitivity at various concentrations ranging from 100 to 0.2 µg/ml.
For S. mutans Transbond plus SEP and Reliance SEP showed antibacterial activity at lesser concentration than Gluma SEP. For L. acidophilus and A. viscosus, Transbond plus SEP showed antibacterial activity at lesser concentration than the other SEPs [Table 2] (original).
| S. mutans | L. acidophilus | A. viscosus | |
|---|---|---|---|
| Transbond plus SEP | 12.5 | 3.12 | 6.25 |
| Reliance SEP | 12.5 | 12.5 | 12.25 |
| Gluma SEP | 50 | 6.25 | 50 |
| Acetone | 50 | 25 | 50 |
Discussion
Malocclusion correction by orthodontic treatment normally takes a long duration. During the treatment, the oral environment undergoes a lot of changes such as low pH, alterations in buffer capacity, pH acidity, salivary flow rate, increased retention of food particles, and retentive sites for microorganisms. It is seen that during orthodontic treatment there is increased colonization of microorganisms such as S. mutans, L. acidophilus, and A. viscosus.[5]
Among the several species of bacteria’s isolated from dental plaque, streptococcus, and lactobacillus are considered to be major human dental pathogens. These bacterias together are considered as odontopathogens.[6]
A. viscosus is another opportunistic dental pathogen responsible for the formation of root surface caries and diseases of the periodontium. These bacteria, namely, S. mutans, L. acidophilus, and A. viscosus together are responsible for dental caries, and therefore, these bacteria were chosen as test microorganisms in this study.
Orthodontic treatment involves bonding of brackets on enamel surface of all teeth. The conventional method involves three different agents: an enamel conditioner, a primer solution, and an adhesive resin. But with the introduction of seventh generation primers, the steps involved in the bond procedure has reduced. The newly introduced seventh generation primers are also known as SEP. These current SEPs are focused toward simplifying the application procedure. Combining conditioning and priming into one step results in a reduction in time and cost-effectiveness for clinicians and patients, provided the clinical bond failure rates are not increased significantly.[3]
The main features of the single-step etch/primer bonding systems are that it does not require separate acid-etching and subsequent rinsing; the liquid itself has a component that conditions the enamel surface.[3]
The active ingredient of the SEPs is a methacrylated phosphoric acid ester that dissolves calcium from hydroxyapatite. Rather than being rinsed away, the removed calcium forms a complex and is incorporated into the network when the primer polymerizes. Etching and monomer penetration to the exposed enamel rods are simultaneous, and the depth of etch and primer penetration are identical.
Antibacterial action of primers is an important factor to prevent caries and the periodontal diseases as the duration of orthodontic treatment is prolonged. The acidic monomers found in self-etching adhesives with low pH have demonstrated antibacterial activity. Many authors have suggested that the antibacterial activity of SEPs is due to inherent properties such as pH, viscosity, and diffusion capacity of antibacterial agents.[7-13] SEPs have an acidic pH, and the acidic nature has been considered as a key factor for bacterial inhibition.[9,10,14]
However, there are many studies that have concluded differently as they did not find a significant relation between the acidity of self-etching adhesives and their antibacterial effects.[7,15,16] Some authors have reasoned that bacteria such as L. acidophilus are acid tolerant (acidophilic) bacteria and can survive at low pH, therefore, the antibacterial effects may not be only due to its acidic pH. However, studies done by Korkmaz et al. have also shown that the monomer present in the primer (SEP) has inhibitory action against L. acidophilus.[2]
Studies conducted by Harper and Loesche concluded that the pH values that completely eliminated bacteria over a 3-h period was 2.3 for Lactobacillus casei and 3.0 for S. mutans.[17] In addition, the buffering capacity of dentin can also limit the effects of the acid.[1,18] As many studies have given contradictory results regarding SEPs, therefore, this study was carried out to assess the antibacterial activity of three SEPs, namely, Transbond plus SEP, Reliance SEP, and Gluma SEP.
ADT and MIC methods were selected as a medium to evaluate the antibacterial activity of SEPs against S. mutans, L. acidophilus, and A. viscosus.
The advantage of ADT was that it allows direct comparisons of test materials against the test microorganisms, indicating which test materials had the potential to eliminate bacteria in the local microenvironment.[19]
ADT is one of the techniques used as a standard to measure antibacterial properties. This test was used as it reflects a combination of antibacterial activity and diffusivity of the components from the materials. It allows the components to gradually leach out from materials as a certain period is needed for the antibacterial agents to reach a concentration that will disturb bacterial cells. However, from the results of ADT, it is not possible to distinguish whether the materials exhibit bactericidal or bacteriostatic effects because the inhibition zone production on the agar plate indicates only hindered bacterial growth.[4]
ADT is easier to perform, but the inhibitory properties of solid materials placed on agar surface are dependent on many factors. The zone of growth inhibition produced by the materials depends on the toxicity of the material against bacteria tested,[19] diffusibility and diffusion coefficient of the material across the culture medium used[19] viscosity,[20] acidity, molecular weight, alkyl chain length, concentration of material, and ability of material to wet the agar surface.[4] A material that diffuses more easily in addition to its direct toxicity will provide a larger zone of inhibition.[4] Therefore by taking all this into consideration, ADT was selected to test the antibacterial activity of the selected SEPs.
The results of ADT method in our study showed that Transbond plus SEP had a highest antibacterial activity against all bacteria’s except A. viscosus. This was in accordance with the study done by Jacobo et al.,[2] they suggested that Transbond plus SEP had antibacterial activity, but it was least compared to other SEP’s tested.
As in our study, we have taken different SEPs compared to their study, therefore, our study showed that Transbond plus SEP had the highest antibacterial activity among the SEP’s tested.
The reasons for the antibacterial property might be that soluble antibacterial components were released into the surrounding environment by Transbond plus SEP. The antibacterial effect of Transbond plus SEP may be attributed to dipotassium hexafluorotitanate as it is known to release fluoride. Some dental materials containing fluoride have been shown to be bacteriostatic.[17,21] The methacrylate monomer in Transbond plus SEP has a lower molecular weight and also has lower viscosity value.[20] Being a low viscosity primer, it spreads easily on agar and may show a greater zone of inhibition. Transbond plus SEP also contains 15%–25% water[20] which makes it hydrophilic and thereby increasing its spread of the zone of inhibition. Transbond SEP has pH of about 0.4,[22] which makes it a strong acidic monomer. The primers acidity[9,10,14] along with other factors such as the diffusibility and diffusion coefficient of the material across the culture medium used[19] low viscosity,[22] lower molecular weight of methacrylate and fluoride has been considered a key factor for bacterial inhibition.
Reliance SEP also showed antibacterial activity against all the bacteria’s tested (mainly A. viscosus). This might be attributed to the presence of nitric acid which has a low pH of 2. Another reason might be because of the presence of acrylates 2-metha cryloyloxyethyl phosphate. These acrylates, in general, may show to be reactive and increase the toxicological risk of monomers.[23]
In our study, Gluma SEP did not show any antibacterial activity with any of the bacteria’s tested. This was not in accordance with a study done by Aziz et al.[24] where they concluded that among all the seventh generation DBAs tested, Gluma SEP had a maximum caries-protective effect. As there was the difference in the design of both the studies, the results could not be compared.
The results of ADT concluded that Transbond plus SEP showed greater antibacterial activity than Reliance SEP against S. mutans and L. acidophilus. Reliance SEP showed greater antibacterial activity than Transbond plus SEP against A. viscosus.
ADT widely used to determine the susceptibility of organisms isolated from clinical specimens have their limitations. The assessment of antibacterial action regarding the pathogen needs to be more precise. In addition, the terms “susceptibility” and “resistant” can have a realistic interpretation. Thus, when in doubt, the way to precise assessment is to determine the MIC of the bacteria concerned.
The MIC is defined as the lowest dilution of resin that inhibited bacterial growth as determined by the lack of turbidity. The lowest dilution which showed growth inhibition appeared to be clear. The dilution below this showed turbidity which means that microorganisms were resistant to the primers at that concentration. Accordingly, in our study, Transbond plus SEP and Reliance SEP inhibits the growth of S. mutans at a lower concentration than Gluma SEP. This indicates that lower concentration of Transbond plus SEP and Reliance SEP is required to have antibacterial activity against S. mutans as compared to Gluma SEP.
Transbond plus SEP showed antibacterial activity at lower concentration than Reliance SEP and Gluma SEP against L. acidophilus and A. viscosus. This indicated that lower concentration of Transbond plus SEP is required to inhibit the growth of L. acidophilus and A. viscosus as compared to Reliance SEP. The Gluma SEP may possess antibacterial activity but at a higher concentration than Transbond plus SEP and Reliance SEP. Therefore, MIC test, in general, showed that SEP tested had varying antibacterial effect at different concentrations.
The results of both ADT and MIC test confirms the antibacterial activity of Transbond plus SEP and Reliance SEP. Both the methods showed a positive correlation. Therefore, the results are in accordance with McClatchey who has stated that inhibition halos on ADT is inversely proportional to the MIC, i.e., higher the zone of inhibition lesser is the dilution of SEP required for antibacterial activity.[25]
Over a period of many years, lot of studies have been done to acertain the antibacterial properties of SEPs; many authors have attributed various reasons for antibacterial activity, therefore, results regarding the factors responsible for antibacterial effects vary.
This study was done in a smaller set of samples to see and compare the antibacterial activity between different SEPs at one point of time rather than for a long duration. Hence, it signifies the comparative antibacterial effect between three SEPs and which primer has got better antibacterial effect at that point of time when evaluated. It needs randomized control trials and long-term results to see the duration of antibacterial effect and then the comparison between SEPs. Orthodontist has direct involvement in plaque accumulation so materials which help to prevent plaque accumulation and having antibacterial activity may be helpful.
The antibacterial effect of the self-etching adhesive systems may be clinically restricted to a short time and to the superficial layers of dentin, and they may be considered limited.[26]
There are very few studies related to antibacterial properties of SEPs and their use in clinical practice. Thus, incorporation of antibacterial agents into SEPs may be helpful in preventing caries, but we recommend further research to be carried out to know the extent of clinical usage of these SEPs and their effectiveness. This study may not have direct clinical significance but may help the patient and orthodontist in less plaque aggregation.
Conclusions
Transbond plus SEP and Reliance SEP showed antibacterial activity in vitro against S. mutans, L. acidophilus, and A. viscosus as derived from the results of ADT
Transbond plus SEP and Reliance SEP are preferred SEPs because of their antibacterial activity against the commonly encountered oral microflora (S. mutans, L. acidophilus A. viscosus).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Selection of Streptococcus mutans and lactobacilli in an intra-oral human caries model. J Dent Res. 1984;63:1197-200.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro study of the antibacterial properties and microbial colonization susceptibility of four self-etching adhesives used in orthodontics. Eur J Orthod. 2014;36:200-6.
- [Google Scholar]
- Current Principles and Techniques (5th ed). Philadelphia: Elsevier; 2012. p. :731-2.
- In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent Mater. 2006;22:527-32.
- [Google Scholar]
- Effect of orthodontic treatment on saliva, plaque and the levels of Streptococcus mutans and Lactobacillus. Med Oral Patol Oral Cir Bucal. 2010;15:e924-9.
- [Google Scholar]
- Comparison of the antibacterial activity of different self-etching primers and adhesives. J Contemp Dent Pract. 2008;9:57-64.
- [CrossRef] [Google Scholar]
- Comparison of antibacterial activity of simplified adhesive systems. Am J Dent. 2002;15:356-60.
- [Google Scholar]
- Antibacterial properties of self-etching dental adhesive systems. J Am Dent Assoc. 2007;138:349-54.
- [Google Scholar]
- Antibacterial activity of different generation dentin-bonding systems. Quintessence Int. 2005;36:339-44.
- [Google Scholar]
- Antibacterial activity of dentine primer containing MDPB after curing. J Dent. 1998;26:267-71.
- [Google Scholar]
- Antibacterial activity of cured dental resin incorporating the antibacterial monomer MDPB and an adhesion-promoting monomer. J Biomed Mater Res. 1998;39:511-5.
- [CrossRef] [Google Scholar]
- In vivo antibacterial effects of dentin primer incorporating MDPB. Oper Dent. 2004;29:369-75.
- [CrossRef] [Google Scholar]
- Effect of dentin on the antibacterial activity of dentin bonding agents. J Endod. 2004;30:352-8.
- [Google Scholar]
- Bacterioclastic action of dodecylpyridinium iodide against Escherichia coli K12W3110. J Antibacterial Antifungal Agents. 1994a;22:461-8.
- [Google Scholar]
- Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dent Mater. 2003;19:313-9.
- [Google Scholar]
- Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent. 2009;37:289-96.
- [CrossRef] [PubMed] [Google Scholar]
- Adhesion of Streptococcus mutans to different types of brackets. Angle Orthod. 2007;77:1090-5.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of self-etching primers on bond strength – Are they reliable? Angle Orthod. 2003;73:64-70.
- [Google Scholar]
- Antibacterial effect of self-etching adhesive systems on Streptococcus mutans. Restor Dent Endod. 2014;39:32-8.
- [Google Scholar]
- Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19:449-57.
- [CrossRef] [Google Scholar]
- Antibacterial effects of several current orthodontic materials against Streptococcus mutans. West Indian Med J. 2012;61:821-5.
- [Google Scholar]
- Shear bond strength of brackets rebonded with a fluoride-releasing and -recharging adhesive system. Angle Orthod. 2009;79:564-70.
- [CrossRef] [Google Scholar]
- Chemical aspects of self-etching enamel-dentin adhesives: A systematic review. Dent Mater. 2005;21:895-910.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of caries-protective effect of three recent dentin bonding agents on demineralization of root surface: An in vitro study. J Res Dent. 2016;4:42-7.
- [Google Scholar]
- Clinical Laboratory Medicine (2nd ed). Philadelphia: Lippincott Williams & Wilkins; 2002. p. :1225.
- Antibacterial activity of various self-etching adhesive systems against oral streptococci. Oper Dent. 2010;35:448-53.
- [CrossRef] [PubMed] [Google Scholar]






