Translate this page into:
Effects of maxillomandibular advancement surgery on a skeletal Class III patient with obstructive sleep apnea: A case report

*Corresponding author: Ghaddy AlSaty, Department of Orthodontics, West Virginia University School of Dentistry, Morgantown, West Virginia, United States. ghadysaty@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: AlSaty G, Burns M, Ngan P. Effects of maxillomandibular advancement surgery on a skeletal Class III patient with obstructive sleep apnea: A case report. APOS Trends Orthod 2021;11(2):161-8.
Abstract
This case report describes the successful surgical treatment of a patient diagnosed with obstructive sleep apnea (OSA). A 55-year-old Caucasian male patient with a body mass index (BMI) of 25.6 kg/m2 sought treatment with a chief concern of excessive daytime sleepiness and fatigue. An initial polysomnography report showed moderate OSA with an apnea-hypopnea index (AHI) of 21.2 events/h, and Epworth Sleepiness Score (ESS) of 12/24. The patient was initially prescribed with CPAP treatment but was unable to tolerate treatment after a few months. Clinical and radiographic examination revealed a concave facial profile with maxillary retrognathism. Intraoral examination revealed generalized gingival recession, missing upper lateral incisors and lower first premolars, anterior crossbite, and maxillary transverse deficiency with bilateral posterior crossbite. The lateral cephalogram showed a narrow posterior airway space at the level of the base of the tongue. The patient was treated with maxillomandibular advancement (MMA) surgery to improve airway obstruction. Results showed balanced facial esthetic and stable occlusion with a complete resolution of the patient’s OSA and a post-operative improvement of AHI from 21.2 to 0.7 events/h and ESS from 12/24 to 3/24. The lowest oxyhemoglobin saturation during sleep was improved to 97%, and the BMI decreased from 25.6 to 25.2 kg/m2. These results suggest that MMA surgical procedure can be used as a definitive treatment for patients with maxillomandibular deficiency and OSA.
Keywords
Maxillomandibular advancement surgery
Obstructive sleep apnea
CBCT
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by obstructive events of the upper airway during sleep, which can be associated with clinical signs and symptoms such as snoring, excessive daytime sleepiness, and fatigue.[1] It is estimated that OSA affects 5–20% of the general adult population, though some authors report figures of up to 26%. Nevertheless, the statistics show that over 50% of all cases go undiagnosed and this disorder is 3 times more common in men than in women.[2] The prevalence rates are higher in certain populations, such as obese patients and post-stroke patients.[3] Class III malocclusion patients make up a large proportion of those seeking surgical-orthodontic treatment due to esthetic and functional problems. These patients are usually treated by surgical procedures such as mandibular setback or bimaxillary orthognathic surgery together with a maxillary procedure. Several investigators reported on narrowing of the posterior airway space after bimaxillary surgery and these patients may suffer from sleep apnea obstruction after surgical procedure.[4,5] A study reported on post-operative narrowing of the upper airway and a reduction of total airway volume (TAV) causing snoring and OSA in Class III subjects following bimaxillary surgery.[6] However, recent studies substantiated that the maxillomandibular advancement procedure (MMA) is an effective treatment in improvement of OSA regardless of age, craniofacial anomalies, weight, body habitus, or medical history.[7,8]
Continuous positive airway pressure (CPAP) therapy is the first line of treatment for patients with OSA syndrome. The use of CPAP prevents upper airway collapse, relieves symptoms, and decreases cardiovascular events. However, for various reasons, this treatment has poor compliance.[9] Mandibular advancing devices (MADs) have been used for OSA patients who cannot tolerate CPAP therapy for OSA management.[3,10] These appliances are intended to hold the mandible or the associated soft tissues forward, resulting in an increased caliber of the upper airway at the oropharyngeal level. Although relief of OSA symptoms may be evident over the short term in many of these patients, there were unwanted side effects that MADs can have on their patient’s occlusion over the long term.[3]
MMA surgery is a clinically effective and safe treatment for most patients with moderate-to-severe OSA as demonstrated by significant decreases in apnea–hypopnea index (AHI), diastolic blood pressure, and subjective sleepiness, with concomitant significant improvements in quality of life.[9] MMA surgery should be considered as the alternative treatment of choice for patients with severe OSA who cannot fully adhere to CPAP therapy.[11] The use of cone-beam computed tomography (CBCT) has been used in diagnostic and morphometric analysis of the hard and soft tissues in routine orthodontic treatment planning. However, there are certain limitations in using these images in the diagnosis of OSA. CBCT provides no information on neuromuscular tone, susceptibility to collapse, or actual function of the airway.[3]
Polysomnographic (PSG) study is an essential part of the surgical workup and must be performed before and after surgery; otherwise, the patient’s PSG parameters and surgeon’s personal success rate will not be appreciated.[12] The severity of OSA is classified by the AHI. It is considered mild when the number of events per hour is between 5 and 20, moderate with 20–35 events/h, and severe when the AHI is over 35. An AHI of 5 or under is considered normal in an adult.[13] Another parameter used for surgical workup is the Epworth sleepiness scale which is designed to measure sleep propensity in a simple and standardized way. Participants completed the Epworth Sleepiness Score (ESS) (0–24) with scores >10 representing excessive daytime sleepiness.[14]
The aim of this case report was to present the short-term changes in the oropharyngeal region in a Class III patient diagnosed with maxillomandibular deficiency and moderate OSA treated with MMA surgery.
DIAGNOSIS AND ETIOLOGY
A 55-year-old Caucasian male patient with a body mass index (BMI) of 25.6 kg/m2 was presented to one of the investigator’s orthodontic offices. The patient’s main complaints were excessive daytime sleepiness and fatigue. The overnight PSG was performed before treatment (T1) and immediately after MMA surgery (T2) using a digital collection system (VIASYS® 9.1 D) according to the manual of the American Academy of Sleep Medicine. The initial report showed a moderate OSA with an AHI of 21.2 events/h and an ESS of 12/24. Initial treatment with CPAP was prescribed but the patient could not tolerate treatment after a few months.
During the clinical and radiographical examination, a concave facial profile with maxillary retrognathism was observed. Intraoral examination revealed a generalized gingival recession, mild dental crowding, anterior crossbite, and maxillary transverse deficiency with a bilateral posterior crossbite. A dental history revealed that the patient had previous orthodontic treatment with upper canine substitution due to missing upper laterals and lower first premolar extraction [Figures 1 and 2]. The lateral cephalogram showed a narrow posterior airway space at the level of the base of the tongue [Figure 3].[15]
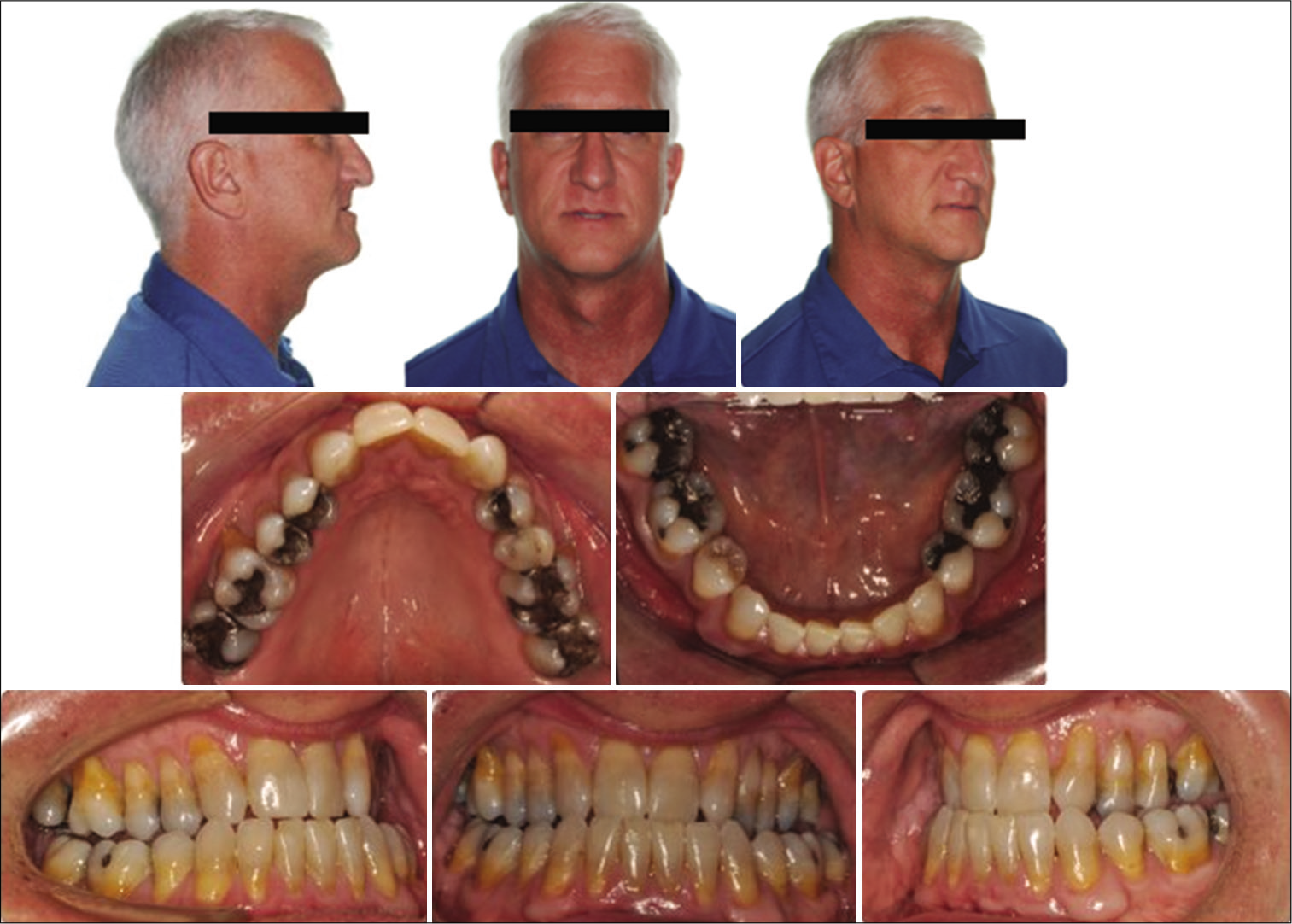
- Pre-treatment extra- and intra-oral photos.
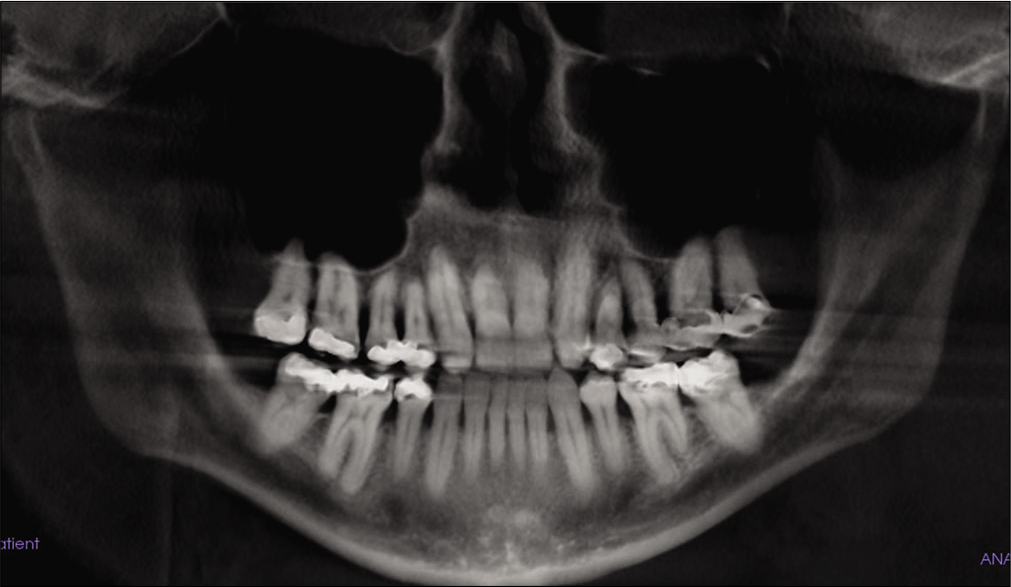
- Pre-treatment panoramic X-ray.
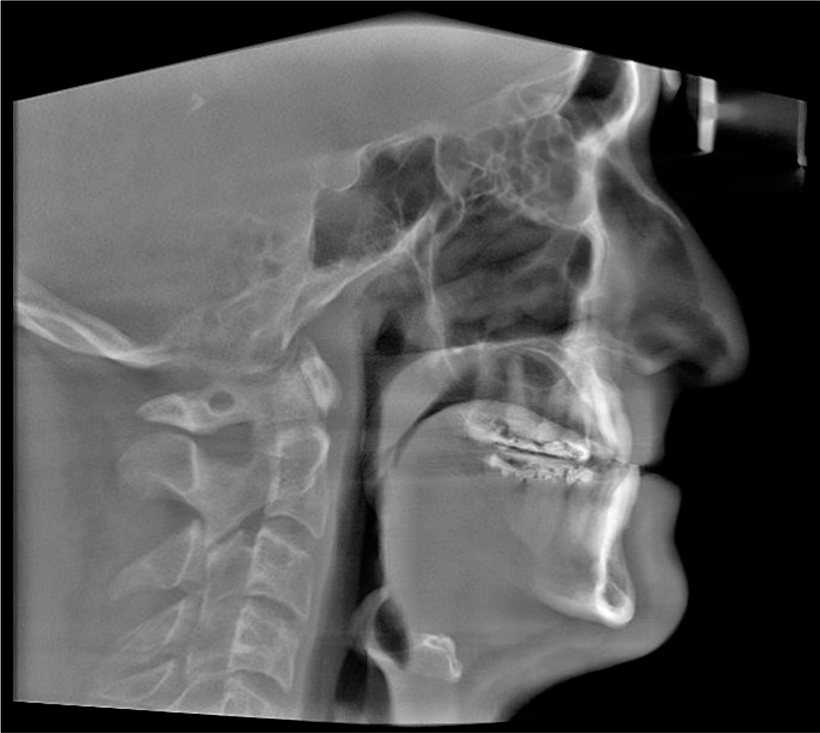
- Pre-treatment cephalometric X-ray.
Treatment objectives
The treatment objectives for this patient included orthodontic, surgical, and prosthodontic treatment to correct the maxillary transverse discrepancy, improve dental alignment, create space for prosthetic replacement of the upper missing lateral incisors, establish overbite and overjet for proper function, and improve soft-tissue profile. In addition, the treatment objectives included enlargement of the posterior airway space to relieve sleep apnea.
Treatment alternatives
Comprehensive orthodontic treatment in conjunction with MMA surgery is the ideal treatment for patients with sleep apnea and maxillomandibular deficiency. Orthognathic surgery is necessary to correct the severe skeletal problems in the transverse and anteroposterior dimensions. However, if the patient declined surgery, the only treatment alternative will be using an oral mandibular advancement device to alleviate sleep apnea symptoms. This treatment will not address the severe skeletal discrepancy and may have side effects such as unnecessary movement of teeth or change of occlusion if the patient wears the appliance for a long period of time. The final option will be no treatment and continue monitoring the patient’s airway with CPAP.
Treatment progress
Pre-surgical orthodontic treatment was carried out for 24 months to prepare the patient for the orthognathic surgery procedure. The surgical procedure included MMA with a combination of two-piece Le Fort I advancement of the maxilla and bilateral sagittal split osteotomies to advance the mandible. The forward movement of the maxilla and mandible was 9 mm and 5 mm, respectively. These movements were limited by the patient’s facial esthetics. Rigid fixation with miniplates was used to retain the surgical results in the maxilla while the forward movement of the mandible was fixed with bicortical screws. The plates and the screws were removed later per the surgeon’s preference. [Figure 4] shows the lateral cephalogram after MMA surgery.
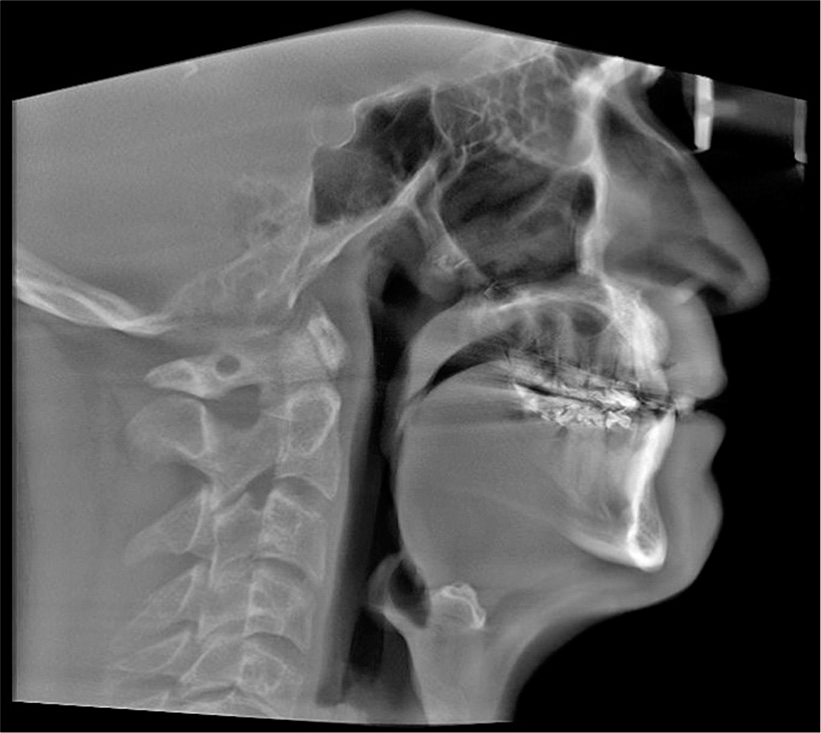
- Post-treatment cephalometric X-ray.
Post-surgical orthodontic treatment was carried out to detail the occlusion. Teeth #8 and 9 were extracted due to loss of bone support and severe gingival recession [Figures 5 and 6]. Space was created initially for prosthodontic replacement of teeth #7 and 10. Several options were discussed with the patients to replace the anterior teeth based on the available space, bone level, and prognosis of treatment. An implant bridge from tooth #7 to 10 will give the patient better long-term esthetical and functional outcomes. A temporary anterior bridge was placed for esthetic reasons.
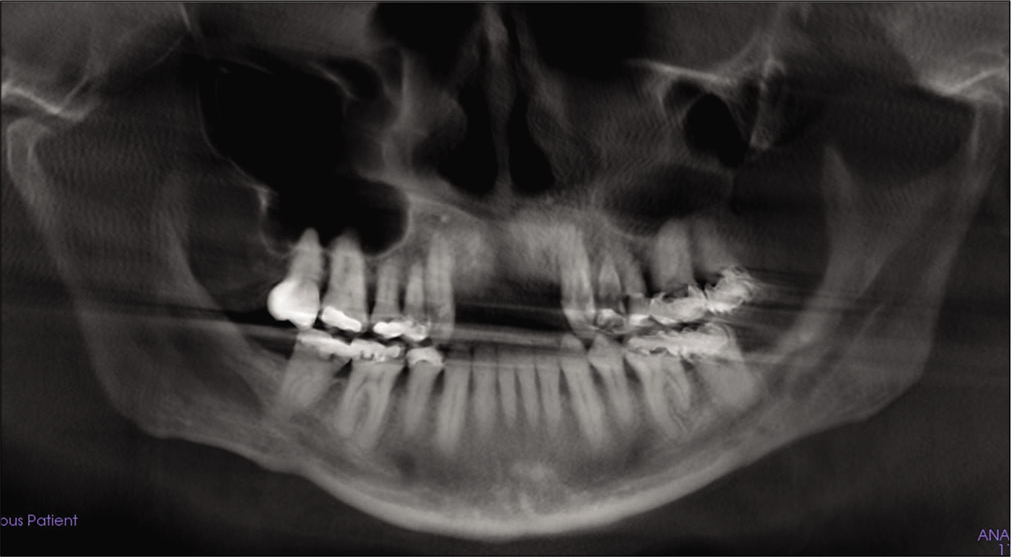
- Post-treatment panoramic X-ray.
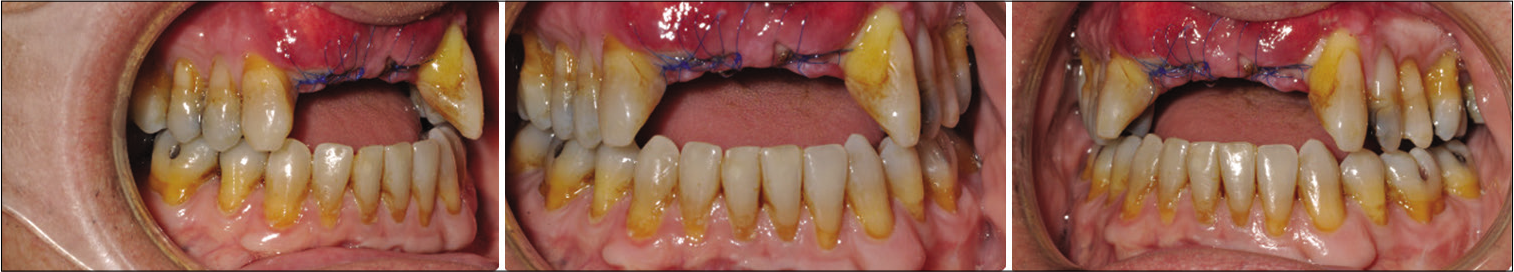
- Post-surgical intraoral photos.
CBCT scans were taken before treatment (T1) and immediately after the MMA procedure (T2). CBCT was performed using the Kodak 9500 unit. All images were calibrated using the following settings: 90 kV, 10 mAs, scanning time of 10.8 s, and an extended height field of view. During image acquisition, the patient was positioned in a natural head posture and in maximum interception position, with lips at rest.
For measurements of PAS, Dolphin (version 11.95, Dolphin Imaging and Management Solutions, Chatsworth, Calif.) software was used to calculate the TAV, airway area (AA), and the minimum cross-sectional area (MCA) selected from predefined structures. The borders of PAS were identified, similar to the method described by El and Palomo,[16] between the palatal plane (ANS-PNS) superiorly extending to the posterior wall of the pharynx and the plane parallel to the palatal plane that passes from the most anterior-inferior point of the third cervical vertebrae (C3) and the base of the epiglottis inferiorly [Figure 7]. TAV, AA, and MCA measurements of the airway were then calculated using a specific analysis tool in Dolphin for the patient in T1 [Figure 8] and T2 [Figure 9]. Figure 10 shows the skeletal superimposition of the 2 time periods T1 and T2.
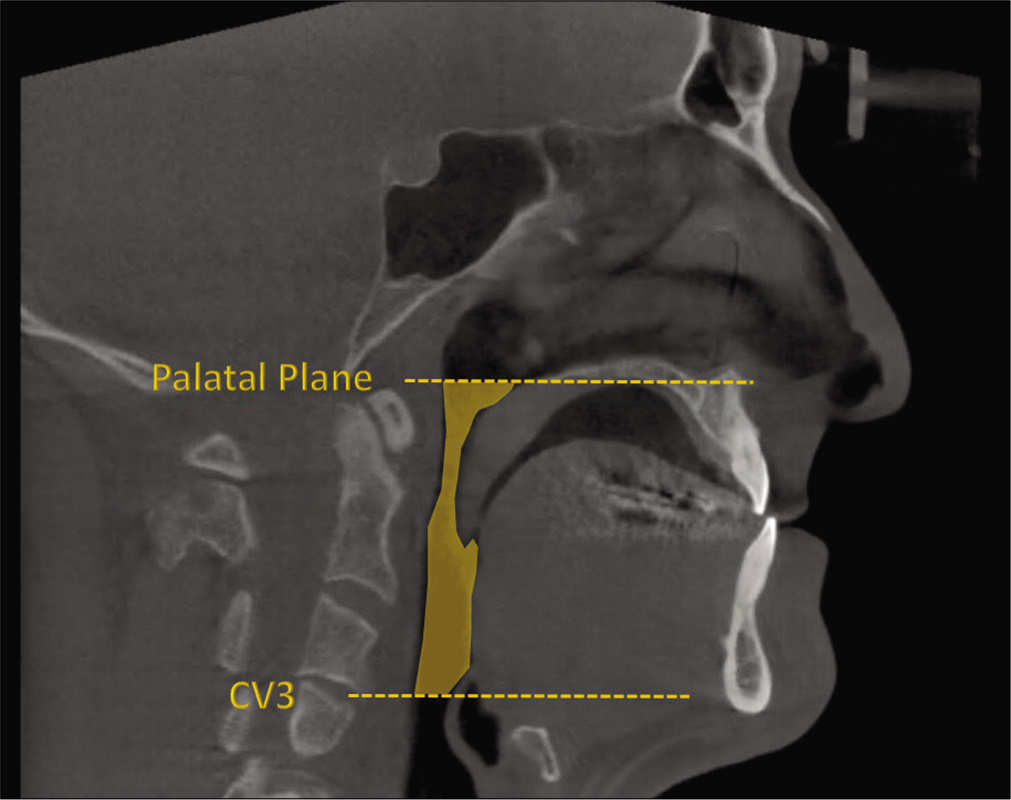
- CBCT landmarks.
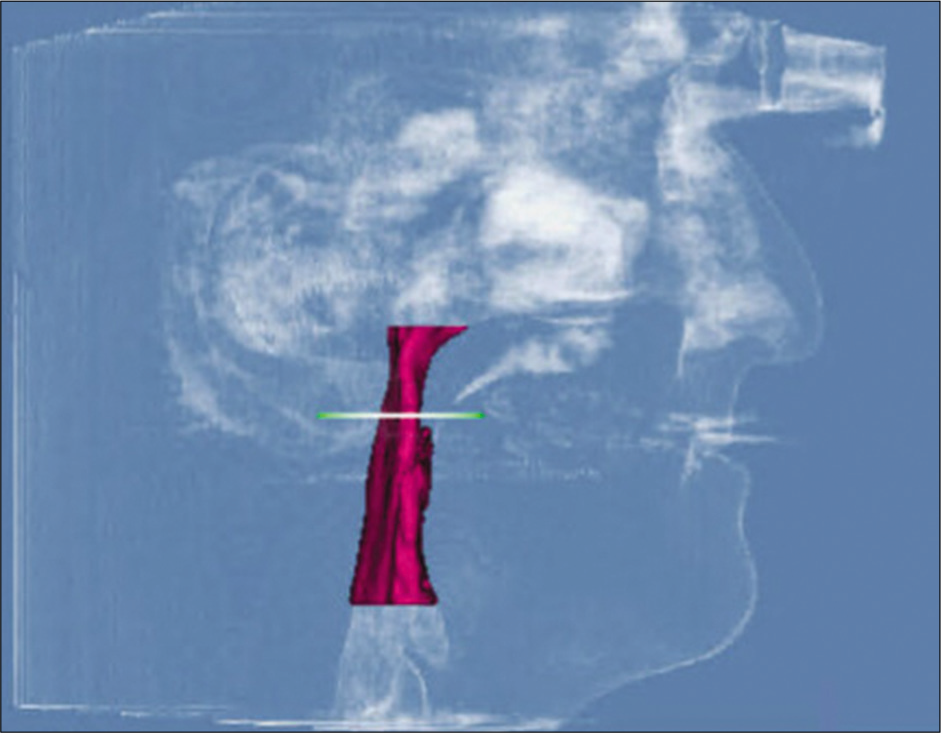
- Pre-treatment airway measurements.
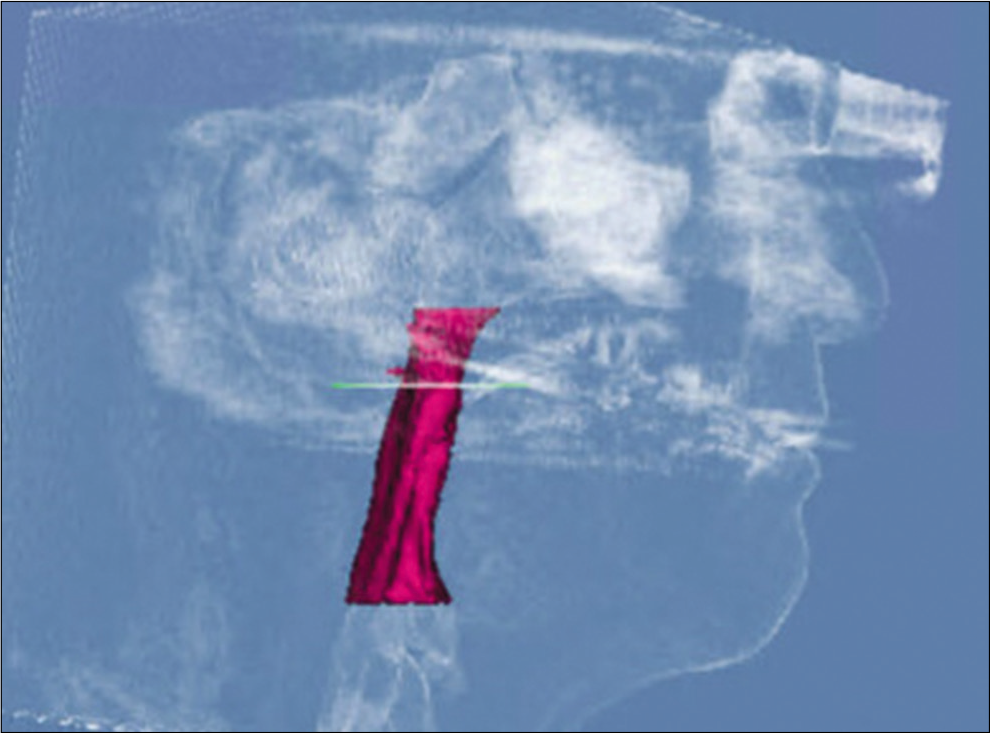
- Post-treatment airway measurements.
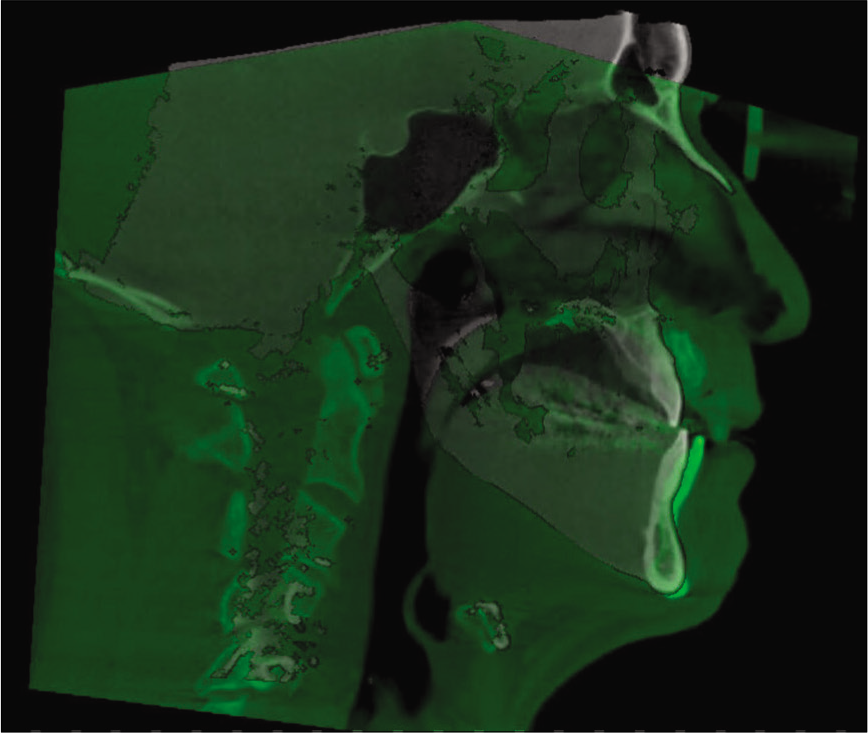
- 3D skeletal superimposition.
Treatment results
On completion of treatment with MMA surgery and orthodontic treatment, correction in the transverse, anteroposterior, and vertical dimensions was achieved. Pre- and post-treatment upper model analysis shows 6 mm of maxillary skeletal expansion gained after treatment in the first premolars and first molars area [Figure 11]. The anterior crossbite was corrected and a stable occlusion was obtained [Figure 12]. The occlusion was acceptable with positive overbite and overjet and with the left buccal side slightly end-on. Figure 13 shows the two-dimensional (2D) superimposition of the two time periods T1 and T2 [Table 1].
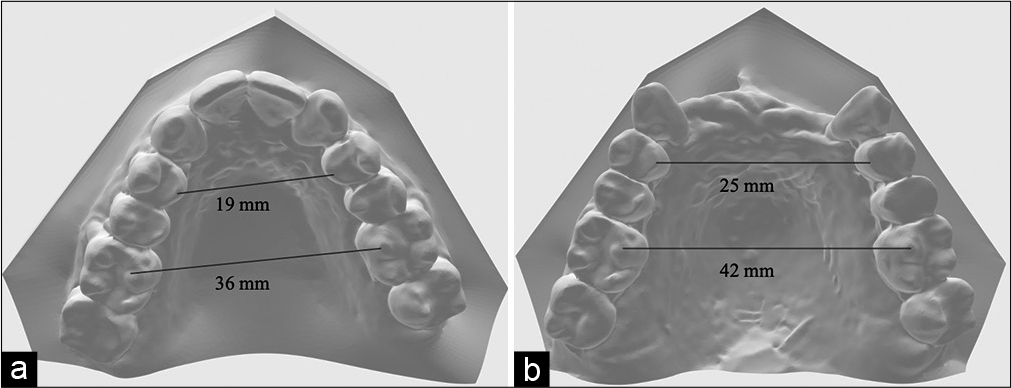
- (a) Pre-treatment, (b) post-treatment upper models.
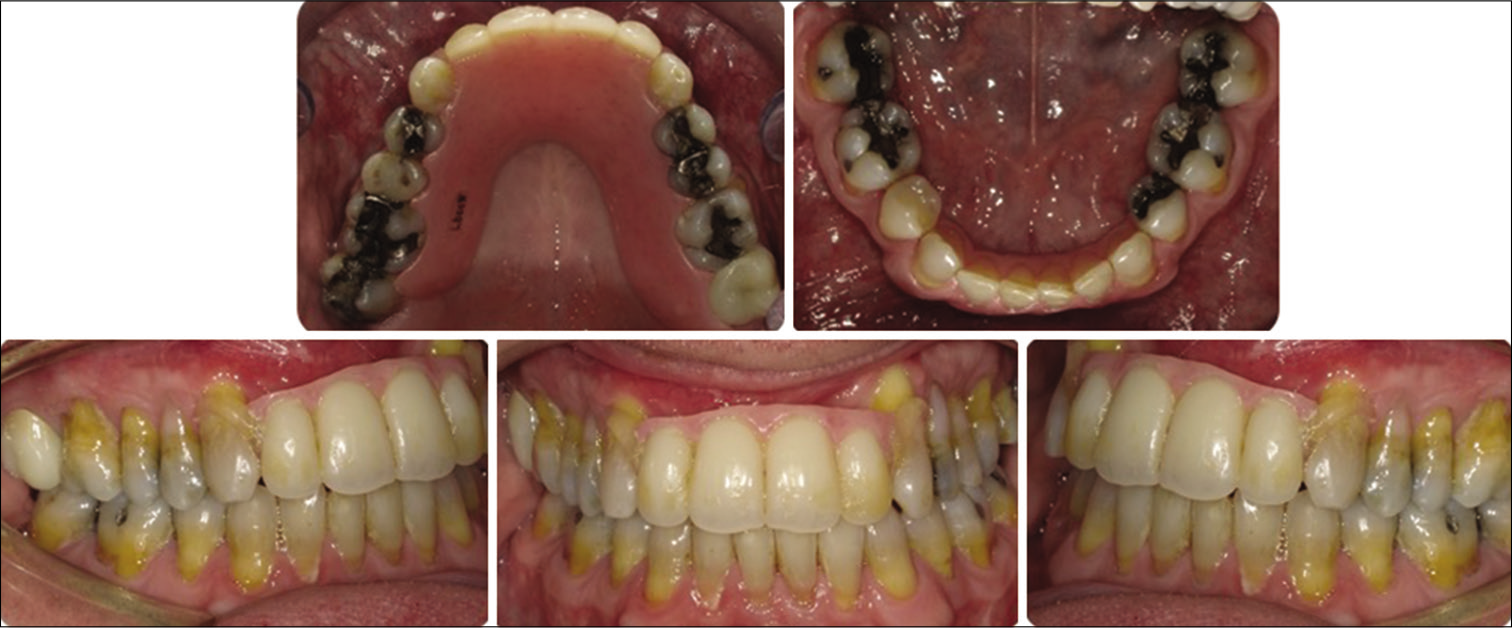
- Post-treatment intraoral photos.
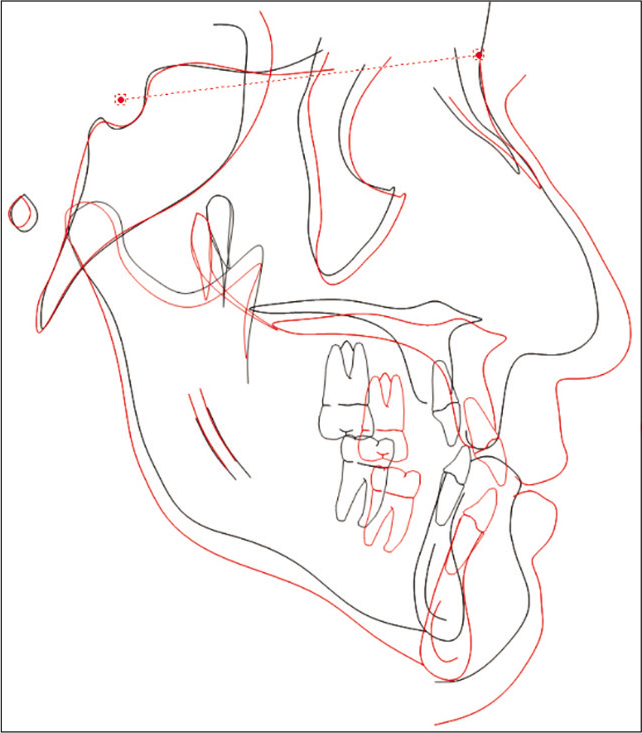
- 2D superimposition.
| Norms | T1 | T2 | |
|---|---|---|---|
| Skeletal | |||
| SNA | 82 | 78.5 | 83.7 |
| SNB | 80 | 78.8 | 81.3 |
| ANB | 2 | –0.3 | 2.4 |
| MP-SN | 33 | 36.9 | 36.9 |
| FMA | 23 | 26.80 | 29.7 |
| Dental | |||
| U1-SN | 103 | 87.6 | 104 |
| L1-MP | 95 | 82.9 | 88.6 |
| Soft tissue | |||
| Upper lip to E plane, mm | –4 | –10 | –4 |
| Lower lip to E plane, mm | –2 | –5 | –3 |
Two months after surgery, another PSG study was performed. Based on the surgical criteria for successful treatment which includes >50% reduction of baseline AHI and a post-treatment AHI <20,[17] There was a complete resolution of the patient’s OSA with a post-operative AHI of 0.7 events/h and an ESS of 3. The lowest oxyhemoglobin saturation during sleep was elevated to 97%, and the BMI decreased to 25.2 kg/m2 [Table 2].
| T1 | T2 | Change in % | |
|---|---|---|---|
| Airway | |||
| TAV, mm≥ | 18373 | 25409 | 27.70 |
| AA, mm2 | 570 | 709 | 19.60 |
| MCA, mm2 | 131 | 277 | 52.70 |
| PSGs | |||
| AHI | 21.2/hr | 0.7/hr | –96.70 |
| ESS | 12 | 3 | –75 |
| BMI, kg/m2 | 25.6 | 25.2 | –1.60 |
TAV: Total airway volume, AA: Airway area, MCA: Minimum cross-sectional area, PSG: Polysomnography, AHI: Apnea–hypopnea index, ESS: Epworth sleepiness score, and BMI: Body mass index
When the pre-operative tomographic images and the images obtained post-surgery were compared, the results showed three-dimensional pharyngeal enlargement with a substantial increase in TAV (18,373–25,409 mm3), which represents a gain of 27.7%, 19.6% increase in AA (590–709 mm2), and 52.7% increase in MCA (131–277 mm2) after the MMA procedure [Table 2].
The soft-tissue profile was improved surgically as both maxilla and mandible were moved anteriorly [Figure 14].

- Post-treatment extraoral photos.
Most importantly, the patient expressed great satisfaction with his smile and improved occlusion.
DISCUSSION
This case report illustrates the short-term effects of comprehensive orthodontic treatment in conjunction with MMA for the treatment of a Class III patient with severe skeletal discrepancy and moderate OSA. Bimaxillary osteotomies have become the standard care for correcting skeletal maxillomandibular discrepancies in adults. Pre-operative orthodontic treatment with correct dental arch alignment and accurate planning of orthognathic surgery improves the skeletal relationship and esthetics.[18] The two-piece segmentation of the maxilla helps to improve maxillomandibular intercuspation and may thus avoid skeletal and orthodontic relapse in the long term.[19] It was proven that MMA is effective in the improvement of OSA regardless of age, craniofacial anomalies, weight, body habitus, or medical history. The use of MMA routinely requires the advancement of the maxillomandibular complex by 8 mm or more to increase the upper airway space to afford its intended results.[20] It was also documented in the literature that the forward movement of the maxilla and/or mandible usually resulted in improvement of facial esthetics.[2] Although the bony movements in our patient with a forward movement of the maxilla (9 mm) and mandible (5 mm) did not achieve the MMA values described in the literature for the successful treatment of OSA, the treatment resulted in an increase in TAV (27.7%), AA (19.6%), and MCS (52.7%) measurements. However, this may not be adequate to establish the magnitude and direction of maxillary or mandibular movement required to completely cure the OSA problem. The three-dimensional increase in the pharyngeal airway (PA) after the MMA procedure agreed with the previous studies.[1,8,19]
According to Holty and Guilleminault, MMA enlarges the PA space by expanding the skeletal framework that the soft-tissue pharyngeal structures and tongue attach to resulting in reduced pharyngeal collapsibility during negative-pressure inspiration, and a statistically significant and clinically relevant reduction in the ESS in all subjects after MMA.[21] Based on surgical success criteria, the results of surgical treatment of OSA are divided into “surgical success” and “surgical cure.” Surgical success is defined as an AHI <20 events/h and >50% reduction in AHI 50% after surgical procedure. Surgical cure is defined as an AHI <5 events/h after surgical procedure.[9] Consequently, there was a complete resolution of the patient’s OSA with postoperative AHI of 0.7 events/h which decreased by 96.7%. Moreover, the ESS improved to 3 (decreased by 75%) as well. These results support the conclusion that MMA surgery has been shown to improve oximetric indicators and the patient’s quality of life measured on the ESS.[13]
In addition, a systematic review by Giralt-Hernando et al.[2] concluded that PSG is considered the gold standard for diagnosing OSA and found a significant association between PA volume gain and AHI reduction. A similar finding was noted by Zaghi et al.[22] who concluded that MMA is a highly effective OSA surgical treatment that is associated with substantial improvements to AHI and respiratory distress index. Long-term follow-up is needed because recurrences of OSA have been noted at 10–15 years after MMA surgery.[23] The follow-up PSG is generally performed between the 4th and 6th months postoperatively. If everything goes well, periodic controls are made every 6 months for the 1st year and then yearly.[21] All patients with OSA should have ongoing, long-term management for their chronic disorder.
Those on chronic therapy (CPAP, MADs, and positional therapy) should have regular, ongoing follow-up to monitor adherence to therapy, side effects, development of medical complications related to OSA, and continued resolution of symptoms. Those with the elimination of OSA (weight loss and surgery) should be monitored for continued risk factor modification and to look for the return of symptoms.[24]
The MMA surgical movements were found to be stable with less than 1 mm of relapse during the follow-up period, which was not clinically significant.[8] According to Lee et al. surgical advancement of the maxillomandibular complex 10 mm for treatment, OSA remains stable at a mean follow-up period greater than 2 years and pre-operative orthodontic treatment does not appear to influence skeletal stability.[10] Another study by Conradt et al. demonstrated that MMA is successful by cephalometric and PSG investigation, and post-operative success has proved to be stable over 2 years.[25] The present case report shows that MMA can be an effective, predictable, and permanent option for treating OSA, with additional benefits of occlusal correction and considerable facial esthetic improvement.
CONCLUSION
The present case report illustrates that MMA can be used as a definitive treatment for patients with maxillomandibular deficiency and OSA in which patients cannot tolerate the use of CPAP. The use of MMA surgery in the present case decreased both the AHI from 21.2 to 0.7 events/h and ESS from 12/24 to 3/24, with a subsequent reduction in daytime sleepiness.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Effectiveness of Maxillomandibular advancement (MMA) surgery in sleep apnea treatment: Case report. Sleep Sci. 2016;9:134-9.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of surgical maxillomandibular advancement upon pharyngeal airway volume and the apnoea-hypopnoea index in the treatment of obstructive sleep apnoea: Systematic review and meta-analysis. BMJ Open Respir Res. 2019;6:1-13.
- [CrossRef] [PubMed] [Google Scholar]
- Obstructive sleep apnea and orthodontics: An American Association of Orthodontists White Paper. Am J Orthod Dentofacial Orthop. 2019;156:13-28.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of mandibular setback surgery on upper airway dimensions and their influence on obstructive sleep apnoea-a systematic review. J Craniomaxillofac Surg. 2015;43:248-53.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of osteotomy procedure and surgical experience on early complications after orthognathic surgery in the mandible. J Craniomaxillofac Surg. 2014;42:e284-8.
- [CrossRef] [PubMed] [Google Scholar]
- The structural changes of upper airway and newly developed sleep breathing disorders after surgical treatment in class III malocclusion subjects. Medicine (Baltimore). 2017;96:e6873.
- [CrossRef] [PubMed] [Google Scholar]
- Maxillomandibular advancement for severe obstructive sleep apnea is a highly skeletally stable long-term procedure. J Oral Maxillofac Surg. 2019;77:1231-6.
- [CrossRef] [PubMed] [Google Scholar]
- Follow-up observation of patients with obstructive sleep apnea treated by maxillomandibular advancement. Am J Orthod Dentofacial Orthop. 2020;158:527-34.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of maxillomandibular advancement in treatment of obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2013;33:43-6.
- [Google Scholar]
- Skeletal stability of patients undergoing maxillomandibular advancement for treatment of obstructive sleep apnea. J Oral Maxillofac Surg. 2015;73:694-700.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term effectiveness and safety of maxillomandibular advancement for treatment of obstructive sleep apnea. J Clin Sleep Med. 2015;11:699-708.
- [CrossRef] [PubMed] [Google Scholar]
- Maxillary, mandibular, and chin advancement: Treatment planning based on airway anatomy in obstructive sleep apnea. J Oral Maxillofac Surg. 2011;69:663-76.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of bimaxillary advancement surgery on the upper airway and on obstructive sleep apnea syndrome: A meta-analysis. Sci Rep. 2018;8:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540-5.
- [CrossRef] [PubMed] [Google Scholar]
- Cephalometric evaluation of the pharyngeal airway space after orthognathic surgery and distraction osteogenesis of the jaw bones. Indian J Plast Surg. 2014;47:346-53.
- [CrossRef] [PubMed] [Google Scholar]
- Measuring the airway in 3 dimensions: A reliability and accuracy study. Am J Orthod Dentofacial Orthop. 2010;137:S50-2.
- [CrossRef] [Google Scholar]
- Redefining success in airway surgery for obstructive sleep apnea: A meta analysis and synthesis of the evidence. Sleep. 2007;30:461-7.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term skeletal and dental stability after orthognathic surgery of the maxillo-mandibular complex in Class II patients with transverse discrepancies. J Craniomaxillofac Surg. 2015;43:1516-21.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study of skeletal and dental stability between rigid and wire fixation for mandibular advancement. Am J Orthod Dentofacial Orthop. 2000;117:638-49.
- [CrossRef] [Google Scholar]
- A 3-dimensional airway analysis of an obstructive sleep apnea surgical correction with cone beam computed tomography. J Oral Maxillofac Surg. 2011;69:2424-36.
- [CrossRef] [PubMed] [Google Scholar]
- Maxillomandibular advancement for the treatment of obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med Rev. 2010;14:287-97.
- [CrossRef] [PubMed] [Google Scholar]
- Maxillomandibular advancement for treatment of obstructive sleep apnea: A meta-analysis. JAMA Otolaryngol Head Neck Surg. 2016;142:58-66.
- [CrossRef] [PubMed] [Google Scholar]
- Large maxillomandibular advancements for obstructive sleep apnea: An operative technique evolved over 30 years. J Craniomaxillofac Surg. 2015;43:1113-8.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-76.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term follow-up after surgical treatment of obstructive sleep apnoea by maxillomandibular advancement. Eur Respir J. 1997;10:123-8.
- [CrossRef] [PubMed] [Google Scholar]






