Translate this page into:
Is gingival biotype a critical determinant of secondary stability of orthodontic mini-implants – A prospective clinical study using resonance frequency analysis

*Corresponding author: Supriya Nambiar, Professor and Head, Department of Orthodontics and Dentofacial Orthopedics, Manipal College of Dental Sciences, Mangalore, Manipal Academy of Higher Education, Manipal - 576 104, Karnataka, India. supriya.nambiar@manipal.edu
-
Received: ,
Accepted: ,
How to cite this article: Pradhan SP, Nambiar S, Shetty S, Shetty S, Jose NP. Is gingival biotype a critical determinant of secondary stability of orthodontic mini-implants – A prospective clinical study using resonance frequency analysis. APOS Trends Orthod 2020;10(4):245-52.
Abstract
Objectives:
The objectives of the study were to determine the association of gingival biotype and secondary stability of orthodontic mini-implants using resonance frequency analysis.
Materials and Methods:
Twenty patients, each receiving two mini-implants, were divided into two groups; thick and thin gingival biotype based on the thickness of gingiva before mini-implant placement. Implant stability was assessed at the time of placement; at the 1st, 2nd, 3rd, and 4th month by resonance frequency analysis. Peri-implant soft-tissue conditions were also examined at each month till 4 months interval using periodontal indices.
Results:
Thick and thin gingival biotype groups showed statistically different implant stability quotient (ISQ). Mini-implants showing signs of failure consistently displayed lesser ISQ. Statistically significant difference was observed in the scores of peri-implant indices of failure and no failure group of mini-implants.
Conclusion:
Mini-implants in thin gingival biotype are more susceptible to failure and peri-implantitis compared to thick gingival biotype. Longitudinal assessment of mini-implant stability may help predict failure so as to avoid long duration and cost of orthodontic treatment.
Keywords
Mini-implant anchorage
Gingival indices
Resonance frequency analysis
Temporary anchorage devices
INTRODUCTION
Increase in efficiency, decrease in treatment time and duration, and easy insertion protocol are one of the few crucial advantages of intraoral temporary anchorage devices.[1] Primary stability of implant is gained by its mechanical engagement in the bone, whereas secondary stability increases gradually as osteoblasts are observed at the implant site.[2] The degree of implant stability depends on gingival and osseous architecture and the condition of the surrounding tissue, that is, bone quality and quantity. Oschenbein and Ross have established a relationship between gingival biotype (thickness of gingiva in faciopalatal dimension) and corresponding underlying osseous architecture.[3] Claffey and Shanley characterized thick gingival biotype having thickness of more than or equal to 2 mm and thin with tissue thickness <1.5 mm.[4] On subjection to various surgical, traumatic, or inflammatory procedures such as implant placement, the two gingival biotypes will behave contrastingly based on their underlying osseous architecture bringing about distinctive patterns of bony remodeling, making it utterly vital to assess gingival biotype before mini-implant placement.
Out of numerous techniques employed to evaluate implant stability, the most recent and popular technique is resonance frequency analysis (RFA) developed by Meredith et al. in 1996.[5] The latest version of the device includes Osstell implant stability quotient (ISQ) device (Osstell® Gothenburg, Sweden). It is a portable handheld device utilizing magnetic frequencies between transducer (A magnetic peg or SmartPeg) and the RFA. RFA results are stated as an ISQ which signifies a standardized unit of stability with values ranging from 1 to 100. Higher ISQ value indicates greater stability whereas low value infers instability.[6] As the degree of implant stability essentially relies on the surrounding soft tissue,[7] it is of utmost importance to establish the effect of gingival biotype on the stability of orthodontic mini-implant for successful implant treatment. Hence, the aim of this study was to correlate gingival biotype with the secondary stability of mini-implants assessed using RFA. Additional objective was to assess the relationship between peri-implant soft-tissue status and the implant stability between the two groups.
MATERIALS AND METHODS
This was a longitudinal prospective single-blind clinical study done on patients undergoing fixed orthodontic treatment and approved by the “Institutional Ethics Committee.” A sample of 20 patients was arrived at by calculating the power of the sample. The 20 patients included were of age between 15 and 30 with healthy gingival and periodontal condition and no signs of horizontal bone loss which was assessed using orthopantomogram or systemic diseases. Periodontally compromised patients with any systemic or bone disorders who were not compliant with proper oral hygiene measures were excluded from the study. During initial examination, patients were randomly allotted into two groups depending on the gingival biotype assessed at the site of mini-implant placement. As this was a single-blinded study, subjects were unaware of their particular gingival biotypes.
Assessment of gingival biotype
Gingival biotype was assessed using a “trans-gingival probing” method following the administration of topical lignocaine 2% gel using an endodontic reamer bearing a rubber stop and a digital caliper [Figure 1]. Depending on the measurements obtained, gingival biotype was divided into thin (<1.5 mm) and thick (≥2 mm) based on the criteria of gingival thickness given by Claffey and Shanley in 1986.[4] The thin group (n = 21) and thick group were (n = 19) in the total of 40 implant sites.
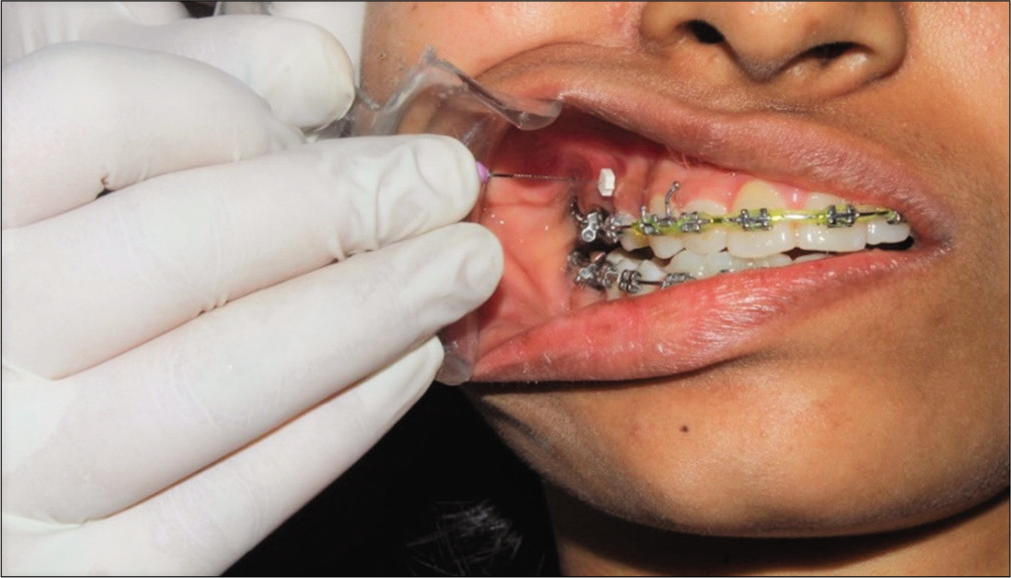
- Assessment of gingival biotype using a transgingival probing method.
Assessment of implant stability
Forty mini-implants (Tapered, self-drilling type, outer diameter 1.3 mm, inner diameter 1.2 mm, length 8 mm [SH1312-08], Ti-6AL-4V alloy, Dentos, Abso Anchor®, Korea) were selected for intraoral placement in 20 subjects. The subjects were randomly selected from a pool of patients who had to have mini-implant placement at the department. Bone density assessment was done as a routine procedure prior to mini-implant placement using cone beam computed tomography (CBCT).
The mini-implant placement site was standardized to maxillary posterior segment between the roots of the second premolar and first molar where bone density of D4 is usually found, hence avoiding bias related to gingival types that can occur with multiple site selection. Later based on gingival thickness at the mini-implant placement site, subjects were divided into thick/thin gingival biotype groups. One mini-implant was placed on either side of maxilla at the decided site under local anesthesia.
The stability was evaluated using RFA with Osstell ISQ device (Osstell® Gothenburg, Sweden). It is suggested that to detect true resonance frequency of an implant in bone, a solid connection between the transducer and implant is crucial; if the connection is not solid, the resonance frequency of the transducer-to-implant interface will not be detected. As mini-implants differ from dental implants in relation to its size, design, surface characteristics, insertion site, as well as insertion protocol, a specially modified SmartPeg is used to establish a solid connection between implant and the transducer. This SmartPeg is available in different sizes for variety of mini-implants. However, for the type of mini-implant used in this study, none of the SmartPeg available were compatible. Hence, an effort was made to establish a direct secure connection between the mini-implant and the transducer using utility wax hardened using cold water spray comparable with a previous study by Su et al.[8] where a bonding adhesive was utilized in an attempt to perform RFA. Utility wax provided enough stability for the magnet with no changes in the ISQ readings taken with or without utility wax. Once the magnet was attached to the mini-implant, a transducer probe was held in a close proximity to the top portion of the magnet and it was activated by a magnetic pulse from the transducer probe. When the instrument captured the response signal from the probe, an audible sound was emitted followed by the display of ISQ value ranging from 1 to 100 [Figure 2]. Uniform force of 150 g which measured using a Dontrix gauge was applied using elastomeric chain to the mini-implant for anterior teeth retraction. Routine oral hygiene instructions were given to all patients.
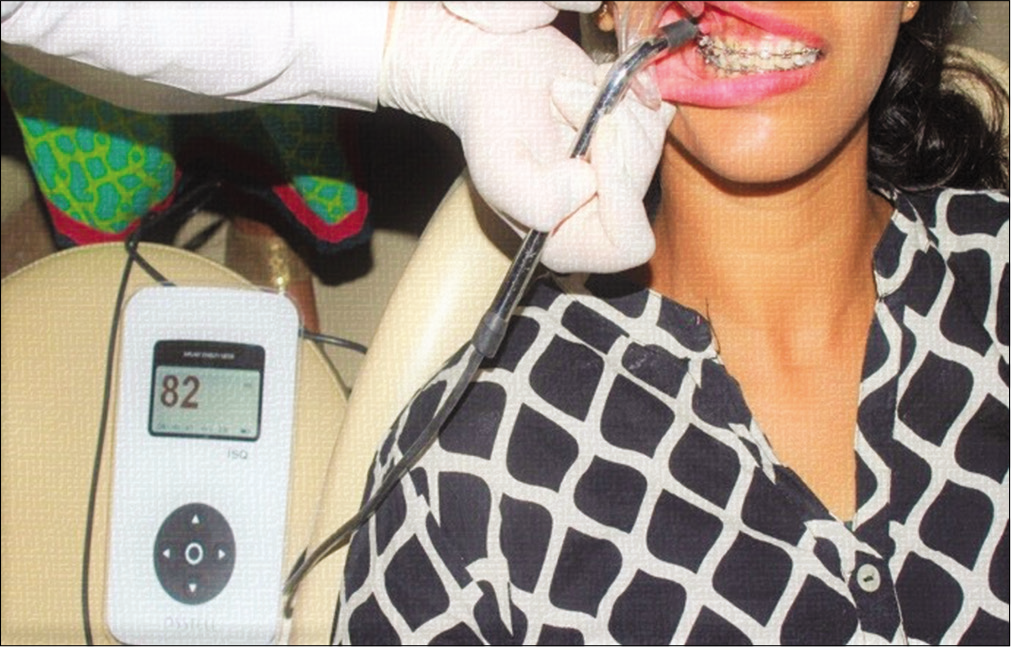
- Assessment of mini-implant stability by resonance frequency analysis using Osstell ISQ device.
The mini-implant stability values were assessed at following intervals: Immediately after implant placement, after loading of mini-implant (ISQ0), at the 1st month (ISQ1), at the 2nd month (ISQ2), at the 3rd month (ISQ3), and at the 4th month (ISQ4). Keeping the baseline ISQ value as a reference, any change the value greater or lower indicated the change in the stability of the mini-implant. A greater value compared to the baseline ISQ indicated better implant stability while a lower value meant loss in stability. No change in the ISQ value reflected constant mini-implant stability.
Assessment of peri-implant soft-tissue status
To assess for inflammation of peri-implant soft tissue, the following indices were used: Modified gingival index (GI) (score 0–2), modified sulcular bleeding index (BI) (score 0–3), and modified plaque index (PI) (score 0–3).[9] These observations were made at baseline that is at the implant placement and at every month till 4 months interval.
Descriptive statistics using SPSS software version 20 (SPSS Inc., Chicago, IL, USA), among a total sample of n = 40, there were thin gingival biotype (n = 21) and thick gingival biotype were (n = 19) with a gender distribution of 4 males and 16 females. [Figure 3] shows the flowchart illustrating the flow of the study.
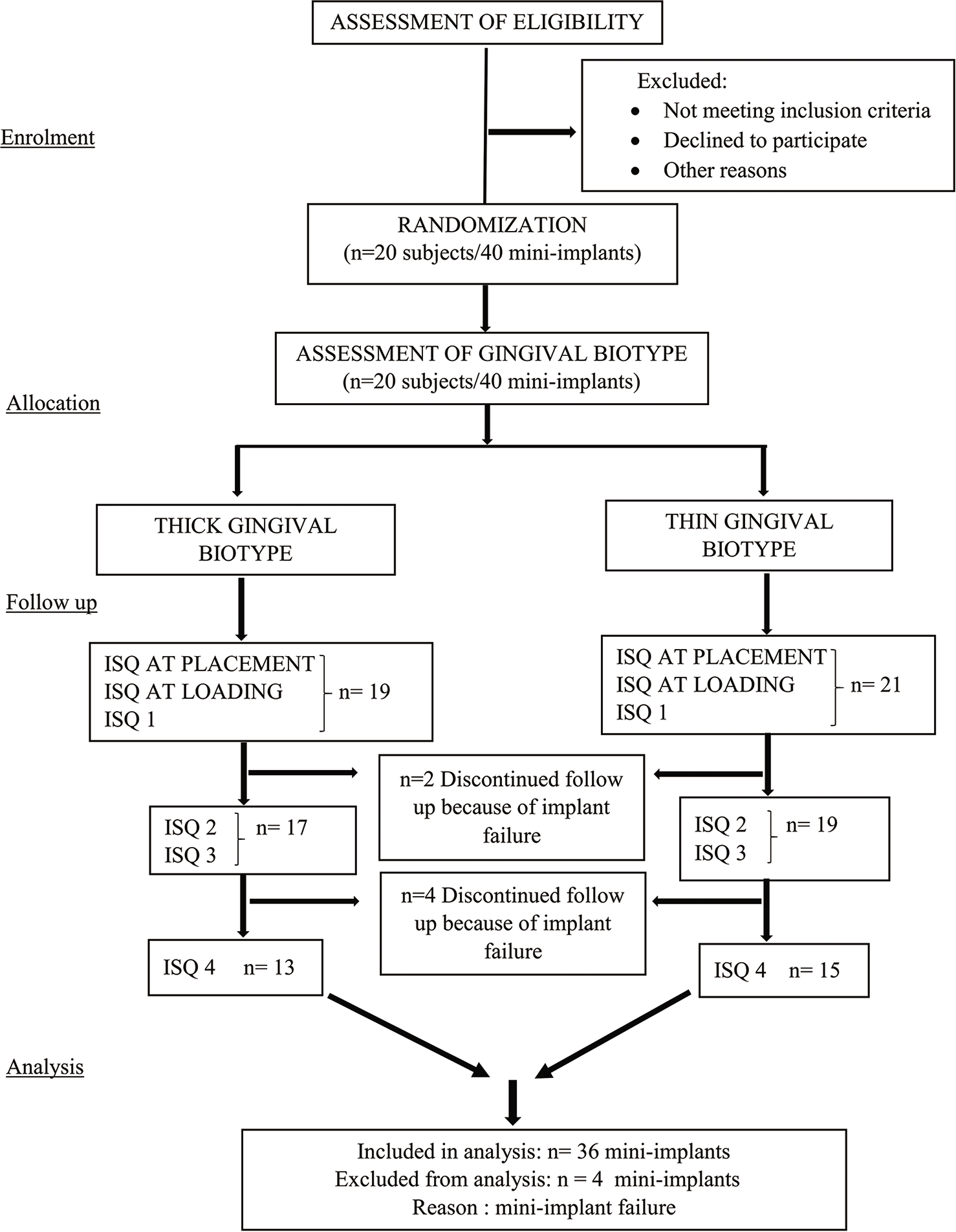
- Flowchart illustrating the flow of the study.
RESULTS
Descriptive statistics (n = 20) for the sample show that 21 mini-implants had thin gingival biotype, whereas 19 mini-implants had a thick gingival biotype with gender distribution as 4 males and 16 females. When comparing the right and the left side, one of the female subjects showed thick gingival biotype on the right side and thin on the left side. Repeated measures of ANOVA test were carried out to compare the stability quotient (ISQ) taken at all intervals shows the mean ISQ values [Table 1] which depicts a slight decrease in the values of ISQ from ISQ 0 at placement to ISQ 4. Greenhouse–Geisser test was done to check intra patient effects among the observations made at different time points shows P = 0.185 (>0.05) showing no statistically significant difference between the mean ISQ values [Table 2]. The mean ISQ values were seen not to differ significantly. Comparison of the two gingival biotypes with ISQ showed decrease in mean ISQ values from baseline till the 4th month with no statistically significant difference. Thick biotype showed significant increase in the ISQ value at the 1st month following slight decrease or stable readings till the 4th month. In contrast, thin gingival biotype showed a significant decrease in the ISQ at the 2nd month with values decreasing from insertion till the 4th month [Figure 4].
| Mean | SD | |
|---|---|---|
| ISQ after placement | 77.96 | 7.633 |
| ISQ after loading | 76.89 | 6.718 |
| ISQ1 | 77.04 | 4.772 |
| ISQ2 | 76.82 | 5.048 |
| ISQ3 | 76.29 | 5.234 |
| ISQ4 | 75.46 | 4.203 |
ISQ: Implant stability quotient
| Tests of within-subjects effects | ||||||
|---|---|---|---|---|---|---|
| Source | Type III sum of squares | df | Mean square | F | P value | |
| Time | Greenhouse–Geisser | 96.601 | 2.255 | 42.830 | 1.712 | 0.185 |
ISQ: Implant stability quotient
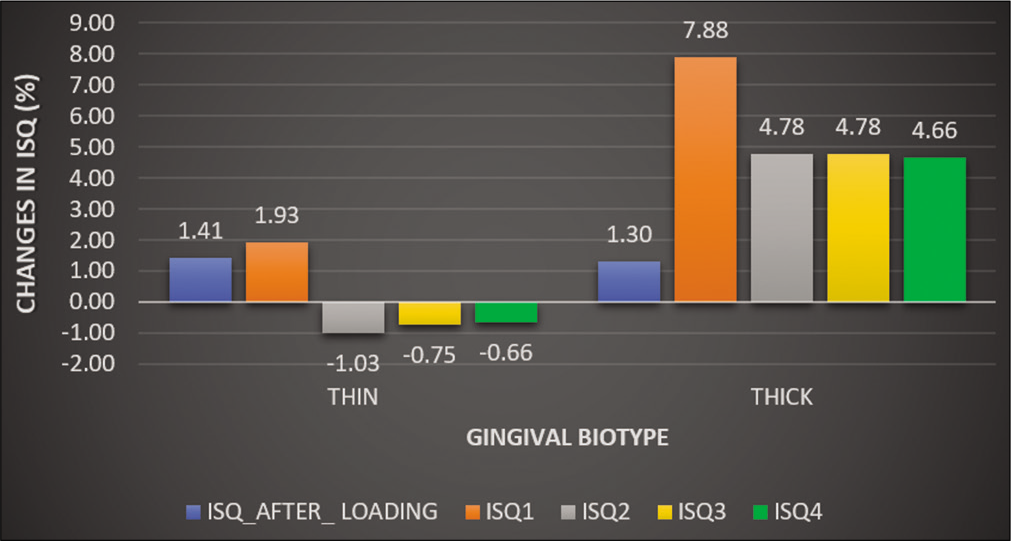
- Comparison of percentage-wise changes in implant stability quotient values overtime in thick biotype versus thin gingival biotype.
As the scores of BI increased from 0 to 2, the mean ISQ values were observed to have decreased. The results for the binary logistic regression test done to assess the parameters responsible for the failure of mini-implants, where the peri-implant soft-tissue inflammation assessed using modified bleeding, gingival, and PI was compared. Comparison between the mean ISQ values after mini-implant placement, after loading and mean ISQ values at the 1st month showed consistently lower ISQ values for the failure group compared to the mini-implants showing no failure [Table 3]. Comparison between failure and no failure group with respect to the scores obtained from periodontal indices shows significantly higher scores for the failure group [Figure 5]. Comparison between the failure and no failure group with respect to ISQ, PI, GI, and BI at the 1st month done using independent Student’s t-test showed ISQ values after placement, after loading and ISQ1 to be higher in no failure group but was statistically insignificant. Comparison of BI-BI1 between the two groups was assessed using independent Student’s t-test which showed higher value in failure group with a statistically significant P = 0.015. The GI and PI-GI1 and PI1 values were higher in failure group with no statistically significant difference. Table 4 shows comparison of two biotypes; thick and thin gingiva done using independent Student’s t-test for all the parameters. BI2 value was higher in thin biotype group which was statistically significant (P = 0.042). P I1 and P I2 were higher in thin group with no statistically significant difference. PI3 and PI4 were found to be higher in thick biotype group with no statistically significant difference. GI2 was found to be higher in thin biotype group in contrast to GI1, GI3, and GI4 with statistically significant value of P = 0.46. Table 4 also shows the comparison of the ISQ after placement between the two groups and that ISQ after placement is higher in thick group which is statistically significant with P = 0.003. Comparison of the ISQ after loading between the two groups shows that ISQ after loading is higher in thick group and is statistically significant with P = 0.002. Higher values of ISQ were observed in thick gingival biotype at ISQ1, ISQ2, ISQ3, and ISQ4 which were statistically insignificant.
| Failure | n | Mean | SD | t | Df | P value | |
|---|---|---|---|---|---|---|---|
| ISQ after placement | No failure | 36 | 77.28 | 7.126 | 0.634 | 38 | 0.53 |
| Failure | 4 | 74.75 | 11.529 | ||||
| ISQ after loading | No failure | 36 | 76 | 6.476 | 0.043 | 3.217 | 0.968 |
| Failure | 4 | 75.75 | 11.442 | ||||
| ISQ1 | No failure | 36 | 75.78 | 4.94 | 5.013 | 3.166 | 0.013 |
| Failure | 4 | 50.5 | 9.95 | ||||
| BI1 | No failure | 36 | 0.11 | 0.319 | −4.732 | 3.206 | 0.015 |
| Failure | 4 | 1.5 | 0.577 | ||||
| PI1 | No failure | 36 | 0.5 | 0.507 | −1.949 | 38 | 0.059 |
| Failure | 4 | 1 | 0 | ||||
| GI1 | No failure | 36 | 0.44 | 0.504 | −2.666 | 3.128 | 0.073 |
| Failure | 4 | 2 | 1.155 |
ISQ: Implant stability quotient, BI: Bleeding index, GI: Gingival index, PI: Plaque index
| Gingival biotype | n | Mean | SD | t | df | P value | |
|---|---|---|---|---|---|---|---|
| ISQ after placement | Thin | 21 | 73.71 | 5.081 | −3.204 | 29.571 | 0.003 |
| Thick | 19 | 80.68 | 8.159 | ||||
| ISQ after loading | Thin | 21 | 72.67 | 3.851 | −3.543 | 25.789 | 0.002 |
| Thick | 19 | 79.63 | 7.747 | ||||
| ISQ1 | Thin | 21 | 72.29 | 9.258 | −0.677 | 38 | 0.503 |
| Thick | 19 | 74.32 | 9.707 | ||||
| ISQ2 | Thin | 19 | 74.47 | 4.427 | −1.354 | 34 | 0.185 |
| Thick | 17 | 76.82 | 5.95 | ||||
| ISQ3 | Thin | 19 | 74.26 | 3.525 | −1.562 | 34 | 0.128 |
| Thick | 17 | 76.82 | 6.106 | ||||
| ISQ4 | Thin | 15 | 74.2 | 2.957 | −1.777 | 26 | 0.087 |
| Thick | 13 | 76.92 | 5.024 | ||||
| BI1 | Thin | 21 | 0.29 | 0.463 | 0.433 | 38 | 0.668 |
| Thick | 19 | 0.21 | 0.631 | ||||
| BI2 | Thin | 19 | 0.21 | 0.419 | 2.191 | 18 | 0.042 |
| Thick | 17 | 0 | 0 | ||||
| BI3 | Thin | 19 | 0 | ||||
| Thick | 17 | 0 | |||||
| BI4 | Thin | 15 | 0 | ||||
| Thick | 13 | 0 | |||||
| PI1 | Thin | 21 | 0.52 | 0.512 | −0.342 | 38 | 0.734 |
| Thick | 19 | 0.58 | 0.507 | ||||
| PI2 | Thin | 19 | 0.32 | 0.478 | 0.525 | 34 | 0.603 |
| Thick | 17 | 0.24 | 0.437 | ||||
| PI3 | Thin | 19 | 0.26 | 0.452 | −0.201 | 34 | 0.842 |
| Thick | 17 | 0.29 | 0.47 | ||||
| PI4 | Thin | 15 | 0.13 | 0.352 | −2.882 | 20.994 | 0.009 |
| Thick | 13 | 0.62 | 0.506 | ||||
| GI1 | Thin | 21 | 0.43 | 0.507 | −1.559 | 38 | 0.127 |
| Thick | 19 | 0.79 | 0.918 | ||||
| GI2 | Thin | 19 | 0.37 | 0.496 | 0.46 | 34 | 0.648 |
| Thick | 17 | 0.29 | 0.47 | ||||
| GI3 | Thin | 9 | 0 | 0 | −2 | 8 | 0.081 |
| Thick | 9 | 0.33 | 0.5 | ||||
| GI4 | Thin | 15 | 0 | 0 | −1.477 | 12 | 0.165 |
| Thick | 13 | 0.15 | 0.376 |
ISQ: Implant stability quotient, BI: Bleeding index, GI: Gingival index, PI: Plaque index
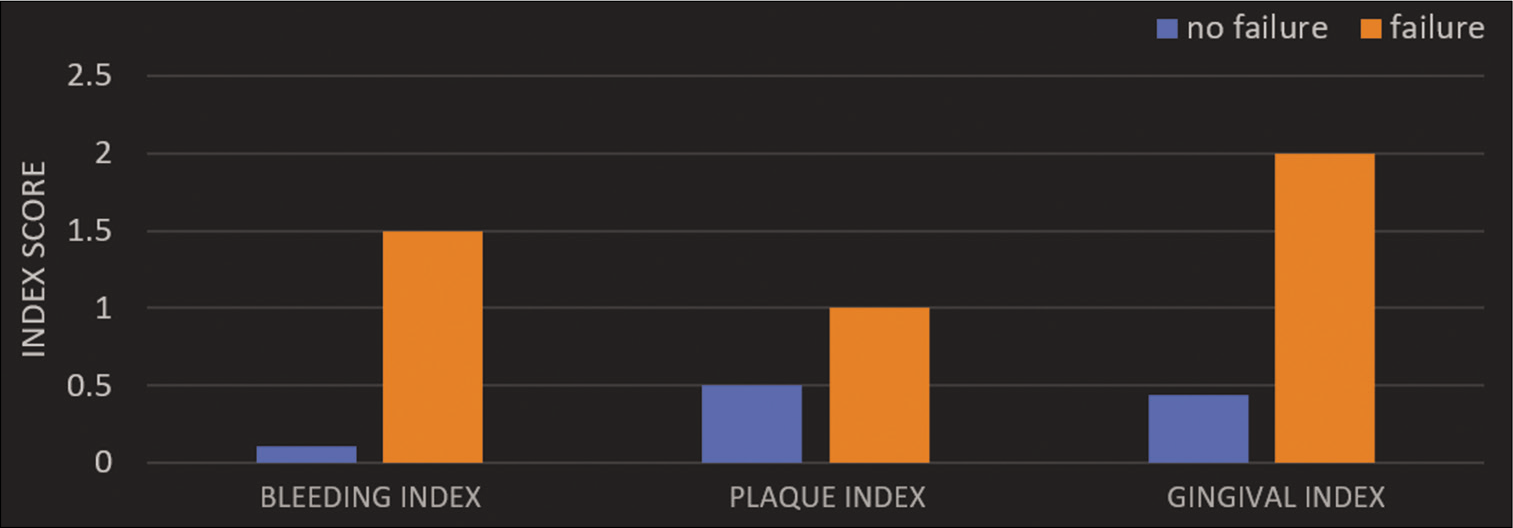
- Comparison of index score value between failure and no failure group.
Comparison of ISQ values for both gingival biotypes shows that the ISQ value at all intervals is lower for thin biotype group compared to the thick biotype group [Figure 6]. Comparison of scores of modified PI evaluated between thin and thick gingival biotype consistently showed high scores at the 1st, 2nd, 3rd, and 4th month interval for thin gingival biotype. In comparison of scores of modified GI were evaluated between thin and thick gingival biotype, thin gingival biotype consistently showed high scores at all-time intervals [Table 4].
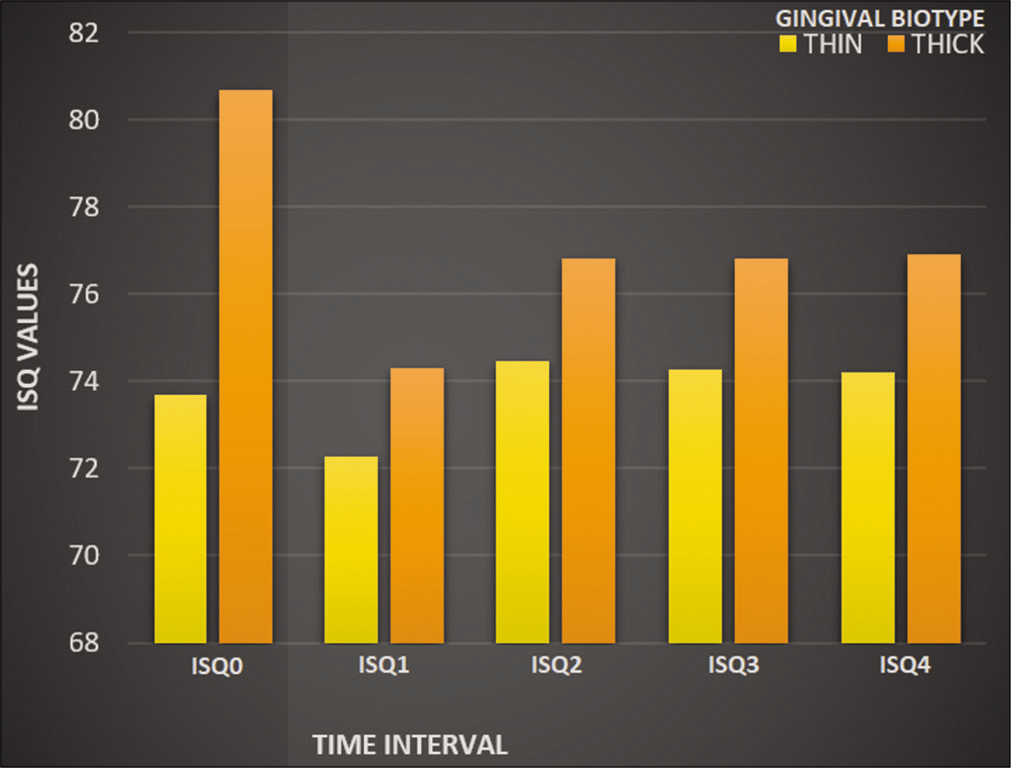
- Comparison of implant stability quotient values for thick and thin gingival biotypes.
DISCUSSION
Mini-implants have proven to be an outstanding alternative to traditional orthodontic anchorage modalities ever since Kanomi and Costa et al. introduced it.[10,11] The identification of the gingival biotype may be crucial in clinical practice as alterations in gingival and osseous architecture have a significant impact on the outcome of mini-implant stability.[12] Transgingival probing to assess gingival thickness developed by Greenberg et al. in 1996 has proven to be a simple, minimally invasive procedure in contrast to CBCT due to disadvantages such as high cost of scans, radiation exposure, and limited resolution due to slice thickness.[13] Due to a random allocation of subjects into the two groups based on the gingival biotype, the present study population shows an unequal gender distribution which disabled comparisons with regard to gender. However, a study by Shah et al. has shown that there is no significant relationship between gender and prevalence of gingival biotype.[14] The main objective of this study was to correlate the implant stability with gingival biotype irrespective of the gender. The total success rate of mini-implants in this study (90%) was comparable to the results obtained by Park et al. who reported success rate from <50% to more than 95%.[15]
Loss of bone to implant communication is regarded as the most definitive reason of mini-implant failures. It alters throughout the primary and secondary stages of stability.[16] Another objective in the present study was to assess the mini-implant stability. Various invasive and non-invasive techniques such as pull-out test, insertion torque analysis, and removal torque assessment have been used to assess implant stability. Turkyilmaz et al. found a significant correlation between the torque value measured at the time of insertion with the underlying bone thickness and density.[17] Extreme insertion torque values, either too high or too low, have also been indicating implant failure. Longitudinal assessment using this technique is impossible without damage to the bone-to-implant interface; it cannot be used to follow implant healing and osseointegration procedures.[18] As non-invasive methods do not disturb the bone-implant interface, they can be used to study the changes in the stability of individual implants overtime. RFA has been proven to be clinically reliable, reproducible, and non-invasive. The RFA measurements done in this study show highest ISQ value immediately after insertion of mini-implants with gradual reduction in the ISQ values. This observation is comparable with findings by Balshi et al., where RFA was done on immediately loaded implant.[19] A possible explanation for this phenomenon is diminishing of the mechanical stability of the mini-implants due to the encompassing hard tissue relaxation explained by bone resorption due to osteoclast activity in the initial healing phase. This supports the idea that primary stability is highest immediately after mini-implant placement and then decreases for a week. The decrease in stability of microimplants during the 1st week can be explained by the physiological processes occurring around the implant. Within 2 h of implant placement, erythrocytes, neutrophils, and macrophages coalesce in a fibrin network; osteoclasts and mesenchymal cells, which appear by day 4, begin removal of bone damaged during mini-implant placement. This leads to the decrease in primary stability observed in the present study which could account for the apparent lower stability observed at the 1st week. Although secondary stability is expected to increase after healing around mini-implant has taken place, there was no statistically significant increase in the mini-implant stability. In this study, 4 out of 40 mini-implants showed signs of failure of which 2 mini-implants belonged to the thin gingival biotype while other 2 mini-implants had a thick gingival biotype. However, higher ISQ values were observed in thick biotype and no failure group at all-time points. According to a study by De Rouck et al., thin gingival biotype shows thin bone quality and a narrow zone of keratinized tissue compared to thick biotype.[12] Placing mini-implants in non-keratinized tissue is thought to be a clinical risk factor. High success rate of mini-implants placed in the keratinized tissue ranging from 91% to 100% has been stated by Lim et al.[20] The peri-implant soft-tissue conditions were also seen to have a significant impact on the implant stability. Thin gingival biotype showed greater scores of gingival, bleeding, and plaque indices, indicating susceptibility of thin soft tissue toward inflammation (GI1, PI1, and BI1). These findings are in accordance with the findings by Miyawaki et al. where titanium screws with inflammation of the peri-implant tissue showed a significantly lower success rate than those without inflammation.[21] Mini-implant failures were observed around the 2nd and 4th month after the insertion [Figure 3]. The indices scores were found to be highest at these points for both thin and thick gingival biotypes correlating the peri-implant soft-tissue inflammation to be the probable cause of the implant failure. The RFA values measured at these points also indicate low implant stability.
CONCLUSION
The present study showed that primary stability decreases gradually after placement and secondary stability increases or remains stable. Mini-implants that failed showed a significant decrease in stability during the first 2 months than mini-implants that remained stable. Mini-implants belonging to the thin gingival biotype showed less stability compared to the ones placed in the thick gingival biotype with marked peri-implant soft-tissue changes. Peri-implant soft-tissue conditions might have a significant impact on the stability of orthodontic mini-implants.
Limitations
In the present study, even though effort was made to customize a suitable and stable connection between the mini-implant and the transducer of the Osstell ISQ device using utility wax which gave consistent and reliable readings of implant stability, the use of SmartPeg would definitely provide superior results and will be clinically more reliable. The results obtained based on the sample of 20 patients included in this study might not be suitable for a large group population making this a preliminary study.
Acknowledgments
We would like to acknowledge Osstell Headquarters, Stampgatan 14, Gothenburg, Sweden, for providing the Osstell ISQ device for the study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Micro-implant anchorage for forced eruption of impacted canines. J Clin Orthod. 2004;38:297-302.
- [Google Scholar]
- Resonance frequency measurement of implant stability in vivo on implants with a sandblasted and acid-etched surface. Int J Oral Maxillofac Implants. 2003;18:641-51.
- [Google Scholar]
- Relationship of gingival thickness and bleeding to loss of probing attachment in shallow sites following nonsurgical periodontal therapy. J Clin Periodontol. 1986;13:654-7.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin Oral Implants Res. 1996;7:261-7.
- [CrossRef] [PubMed] [Google Scholar]
- Measurement of mini-implant stability using resonance frequency analysis. Angle Orthod. 2013;83:230-8.
- [CrossRef] [PubMed] [Google Scholar]
- Factors affecting the clinical success of screw implants used as orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2006;130:18-25.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of self-tapping and self-drilling orthodontic mini-implants: An animal study of insertion torque and displacement under lateral loading. Int J Oral Maxillofac Implants. 2009;24:404-11.
- [Google Scholar]
- The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145-51.
- [CrossRef] [PubMed] [Google Scholar]
- Miniscrews as orthodontic anchorage: A preliminary report. Int J Adult Orthodon Orthognath Surg. 1998;13:201-9.
- [Google Scholar]
- The gingival biotype revisited: Transparency of the periodontal probe through the gingival margin as a method to discriminate thin from thick gingiva. J Clin Periodontol. 2009;36:428-33.
- [CrossRef] [PubMed] [Google Scholar]
- Transgingival probing as a potential estimator of alveolar bone level. J Periodontol. 1976;47:514-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of gingival biotype and its relationship to clinical parameters. Contemp Clin Dent. 2015;6(Suppl 1):S167-71.
- [CrossRef] [PubMed] [Google Scholar]
- Orthodontic Treatment Using Micro-Implant (2nd ed). Seoul: Narae Publishing Inc; 2006. p. 11-15, 398-406
- [Google Scholar]
- Stability changes of miniscrew implants over time. Angle Orthod. 2011;81:994-1000.
- [CrossRef] [PubMed] [Google Scholar]
- Relations between the bone density values from computerized tomography, and implant stability parameters: A clinical study of 230 regular platform implants. J Clin Periodontol. 2007;34:716-22.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanical characteristics of various orthodontic mini-screws in relation to artificial cortical bone thickness. Angle Orthod. 2007;77:979-85.
- [CrossRef] [PubMed] [Google Scholar]
- A resonance frequency analysis assessment of maxillary and mandibular immediately loaded implants. Int J Oral Maxillofac Implants. 2005;20:584-94.
- [Google Scholar]
- Factors associated with initial stability of miniscrews for orthodontic treatment. Am J Orthod Dentofacial Orthop. 2009;136:236-42.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with the stability of titanium screws placed in the posterior region for orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2003;124:373-8.
- [CrossRef] [Google Scholar]






