Translate this page into:
Biodegradation of orthodontic composites by Streptococcus mutans: An in vitro qualitative and quantitative assessment

*Corresponding author: Raoul Bationo, Service de Chirurgie Dentaire, CHU de Bogodogo, Ouagadougou, Burkina Faso, West Africa. raobat10@yahoo.fr
-
Received: ,
Accepted: ,
How to cite this article: Bationo R, Beugré-Kouassi ML, Jordana F, Beugré J. Biodegradation of orthodontic composites by Streptococcus mutans: An in vitro qualitative and quantitative assessment. APOS Trends Orthod 2020;10(4):238-44.
Abstract
Objectives:
The purpose of this study was to evaluate the degradation products of orthodontic composites (Grengloo, Blugloo, Transbond XT, and Transbond LR) by Streptococcus mutans and then to quantify the levels of released bisphenol A (BPA) using gas-phase chromatography and mass spectrometry (GC–MS).
Materials and Methods:
Orthodontic light-cured composite discs were incubated at 37°C in brain heart infusion (BHI) (control group) and in a culture of S. mutans with BHI (test group). Incubation solutions were collected every 48 h in each group and replaced with fresh solutions. These incubation solutions were accumulated and grouped. The assessment of degradation products from composites was done at 1 and 30 days. Detected BPA was then quantified. The limit of quantification was 0.01 μg/mL.
Results:
Degradation products were present at day 30. For the test group, BPA was detected in Blugloo at day 1 (0.38 μg/mL) and triethylene glycol dimethacrylate (TEGDMA) was detected in Grengloo and Transbond LR at day 1.
Conclusion:
S. mutans can hydrolyze long-term orthodontic composites. Monomers such as BPA and TEGDMA may be present in degradation products. It is possible to separate and identify leaching compounds by GC–MS technique.
Keywords
Orthodontic composites
Bisphenol A
Triethylene glycol dimethacrylate
Streptococcus mutans
Gas-phase chromatography and mass spectrometry
INTRODUCTION
Brackets bonding has become a daily act in orthodontics since Newman has proposed in 1965 to paste directly orthodontic brackets using an epoxy resin in replacement of sealing systems.[1]
The composite resins used in dentistry or orthodontics are complex polymers containing a variety of monomers, initiators, activators, stabilizers, plasticizers, and other additives. Two monomers are mainly used in orthodontic adhesive resins: Bisphenol A (BPA) diglycidyl dimethacrylate (Bis-GMA) and triethylene glycol dimethacrylate (TEGDMA). BPA is used as a raw material for the formulation of Bis-GMA.[2]
Several monomers can enter the manufacturing of composite resins. In general, the main used monomer is Bis-GMA. However, its high viscosity prevents optimum handling. It should be diluted with other monomers such as TEGDMA or urethane dimethacrylate (UDMA).[3]
The proportions of the monomers vary considerably according to the products and their clinical use. This is a proprietary trade secret maintained by the manufacturers.
In the intraoral environment, composite resins are exposed to extreme thermal changes, pH variances, mechanical erosion, and degradation occurrence from bacterial and salivary enzymes, which can cause BPA release.[4,5]
Several monomers contained in composite resins (such as BisGMA, ethoxylated bisphenol A dimethacrylate [Bis-EMA], UDMA, and TEGDMA declared by manufacturer) are known to diffuse from partially polymerized materials and to be cytotoxic in vitro.[6,7]
The toxicity of resin-based materials is due to residual monomers as well as to the degradation products linked to the activity of salivary esterases.[8,9]
BPA is an endocrine disruptor with potential toxicity in vitro[10] and in vivo.[11]
Other compounds including TEGDMA and Bis-GMA, released by restorative and bonding composites, also present potential toxicity.[12-19] Infants, young children, and pregnant or lactating women are the most sensitive.[20]
Streptococcus mutans, one of the primary inhabitants present in saliva and at the restoration material-tooth interface, is regarded as the chief etiological agent responsible for dental caries.[21,22]
During the demineralization of the enamel organ, it is possible to observe white opaque lesions of the enamel to the junction between the brackets and the enamel. These lesions are minimal in general and not extended.[23]
The demineralization process is fast and can appear in the 4th week of orthodontic treatment. These lesions are mainly related to inadequate plate control and ill-fitting orthodontic attachments.[24]
In orthodontics, because of the many sites of bacterial retention, the amount of S. mutans is increased during fixed treatments.[25-31]
Streptococcus species have been shown to contain esterases.[32] A study by Lara-Carrillo et al.[27] highlights the change in the saliva flow and the saliva buffer with the bonding of the orthodontic attachments.
It has been shown that the enzyme activity of saliva[33,34] and bacteria[34-36] has an impact on composite resins. This degradation of composite resins is done by an activity that affects the ester links of the organic matrix. These ester links are present in Bis-GMA and TEGDMA. In addition, the release of some products through the degradation of composite resins can have an effect on the growth of some bacteria species of Streptococcus and Lactobacillus.[37]
The purpose of this study was to evaluate the degradation products of orthodontic composites by S. mutans and then to quantify the levels of released BPA using gas-phase chromatography and mass spectrometry (GC–MS).
MATERIALS AND METHODS
Biodegradation experiment
Orthodontic light-cured composites were used for this study [Table 1]. Resin discs (10 mm in diameter and 1 mm in thickness) were prepared following the ISO 10993–12:2012 standard for medical device testing in biologic systems (n = 4 per resin).[38] They were then cured for 20 s using BA Optima 10 LED Curing Light (light intensity 1000 ~ 1200 mW/cm2 and wavelength 420 ~ 480 nm).
S. mutans strain (ATCC 25175 from Kwik-Stik microorganisms) was cultured on LB agar medium supplemented with brain heart infusion (BHI) broth.
The resin discs (n = 2 per resin and group) were incubated in 12-well plates at 37°C [Figure 1]. Each well was filled with 400 μL, either of BHI (control group) or S. mutans in BHI (test group). Incubation solutions were collected every 48 h in each group and replaced with fresh solutions. These incubation solutions were accumulated and grouped. The assessment of degradation products from the composites was done at day 1 and day 30.[35] Bacitracin solution was added to the BHI to achieve a pure culture of S. mutans. The final concentration in bacitracin of culture medium was 0.2 U/mL.
Analytical method
The eluates of incubation solutions were extracted using solid-phase extraction (NH2 cartridge) and then analyzed by gas-phase chromatography and mass spectrometry (Agilent 6890 Series – Agilent 7673). A capillary column 30 m in length, internal diameter of 320 μm, and film thickness of 0.25 μm were used with helium carrier gas at a flow rate of 1.2 mL/min. The column temperature program was set as follows: Initially, 80°C for 1 min, increasing to 150°C at a rate of 20°C/min, and then increasing to 280°C for 2 min at a rate of 10°C per min. The injector temperature was 280°C and the transfer line was 280°C. Mass spectra were obtained using electron impact ionization (69.9 eV, 34.6 μA, 230°C).
| Product (lot) | Resin matrix | Manufacturer |
|---|---|---|
| Grengloo (6623923) | TEGDMA, UDMA, HEMA, Bis-EMA6, GMA, EO-TMPTA, 3-trimethoxysilylpropyl methacrylate | Ormco |
| Blugloo (6556174) | UDMA, Bis-EMA6, GMA, EO-TMPTA, 3-trimethoxysilylpropyl methacrylate | |
| Transbond XT (N921496) | Bis-GMA, Bis-MEPP | 3M |
| Transbond LR (N919866) | Bis-GMA, TEGDMA | |
| Compound | BHI d1 | SM d1 | BHI d30 | SM d30 |
|---|---|---|---|---|
| C7H10N2O2 | X | X | X | X |
| C11H18N2O2 | X | X | X | X |
| C14H16N2O2 | X | X | X | X |
| C15H20N2O2 | X | X | X | X |
| C15H20N2S | X | X | X | X |
| Uric acid C5H4N4O3 | X | X | X | |
| Oleic acid C18H34O2 | X | X | ||
| C12H14N2O2 | X | |||
| C7H12N2O3 | X | |||
| TEGDMA C14H22O6 | X | |||
| BPA C15H16O2 | X | |||
| C21H44 | X | |||
| C17H36 | X | |||
| C20H36O2 | X | |||
| C20H38O2 | X | |||
| Octadecanoic acid C18H36O2 | X | |||
| C20H25NO3S | X | |||
| C20H40O2 | X | |||
| C18H32O2 | X | |||
| C23H32O2 | X | |||
| C5H9NO | X |
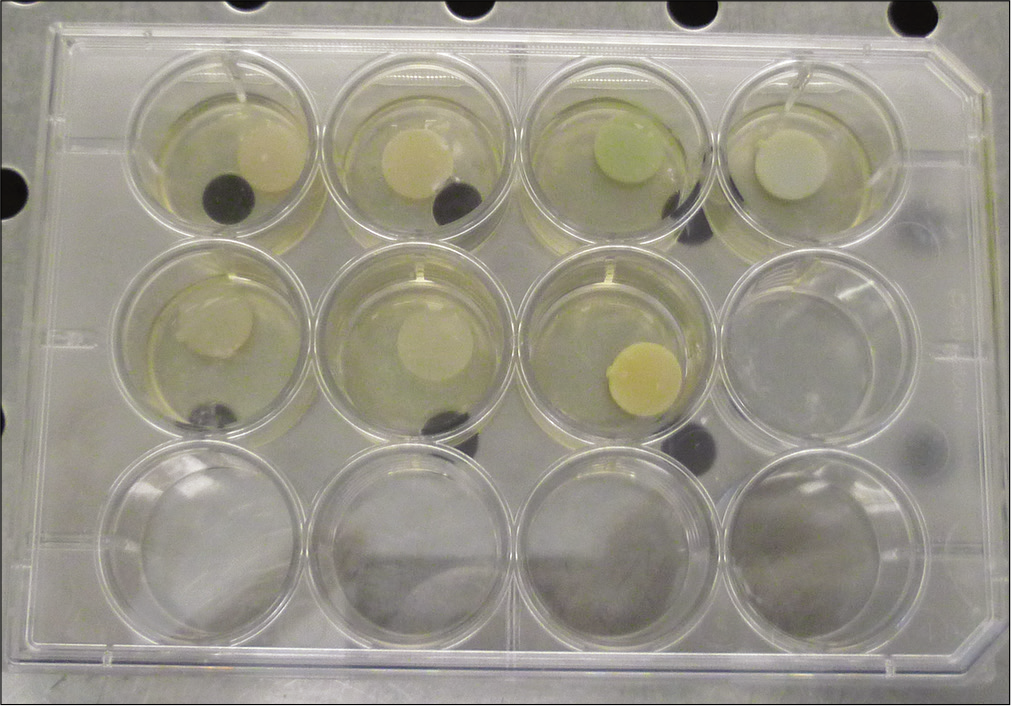
- Resin samples immersed in brain heart infusion.Data were acquired by scan mode and selected ion monitoring (SIM) mode and were processed with MSD ChemStation software.
BPA and TEGDMA were identified by searching for their fragments in SIM mode.
The calibration curve and response factor were computed with reference BPA and caffeine as internal standard. Linear correlation with efficiency of 0.996 was obtained between BPA amount and corresponding peak area. BPA was quantified after his identification. The limit of quantification was 0.01 μg/mL.
RESULTS
Many chemical molecules were identified in tested resin materials [Tables 2 and 3].
For the test group, BPA was detected in Blugloo at day 1 (0.38 μg/mL) [Figure 2]. TEGDMA was present in Grengloo and Transbond LR at day 1 [Figure 3].
DISCUSSION
Resin composites and adhesives are subject to a significant amount of biological breakdown in the oral cavity due to the presence of condensation type bonds within the resin.[39] These bonds, which include esters, urethanes, and amides, are predominantly found in the di-vinyl monomers, and they are all prone to chemical hydrolysis, catalyzed by acids, bases, or enzymes.[40]
| Compound | Grengloo | Blugloo | Transbond XT | Transbond LR |
|---|---|---|---|---|
| C7H10N2O2 | X | X | X | |
| C11H18N2O2 | X | X | X | |
| C14H16N2O2 | X | X | X | X |
| C15H20N2O2 | X | X | X | X |
| C15H20N2S | X | X | X | X |
| Uric acid C5H4N4O3 | X | X | X | X |
| Oleic acid C18H34O2 | X | X | X | |
| C12H14N2O2 | X | |||
| C7H12N2O3 | X | |||
| TEGDMA C14H22O6 | X | X | ||
| BPA C15H16O2 | X | |||
| C21H44 | X | |||
| C17H36 | X | |||
| C20H36O2 | X | X | X | |
| C20H38O2 | X | |||
| Octadecanoic acid C18H36O2 | X | X | X | X |
| C20H25NO3S | X | |||
| C20H40O2 | X | |||
| C18H32O2 | X | |||
| C23H32O2 | X | |||
| C5H9NO | X |
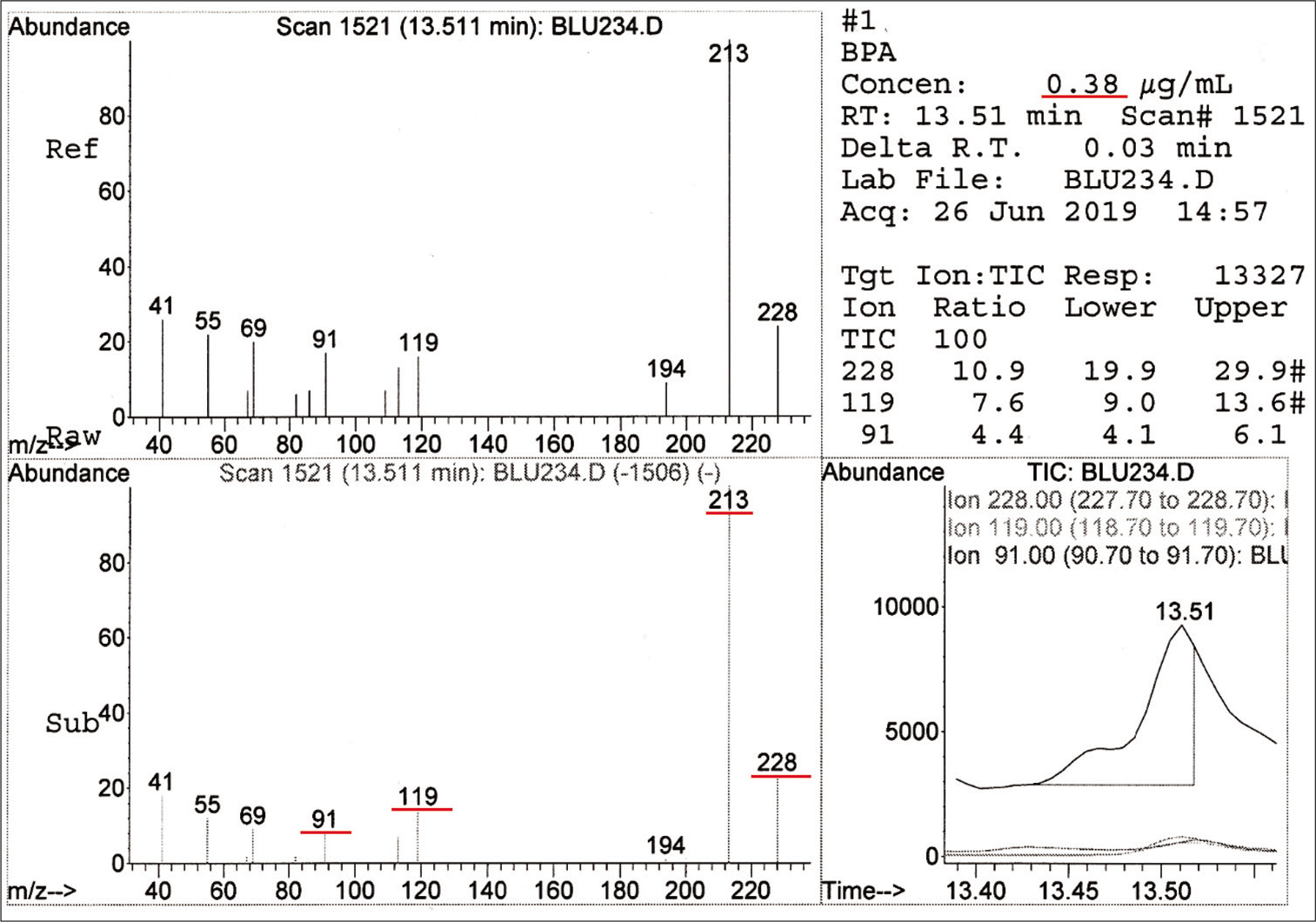
- Spectrum and concentration of bisphenol A detected in Blugloo.
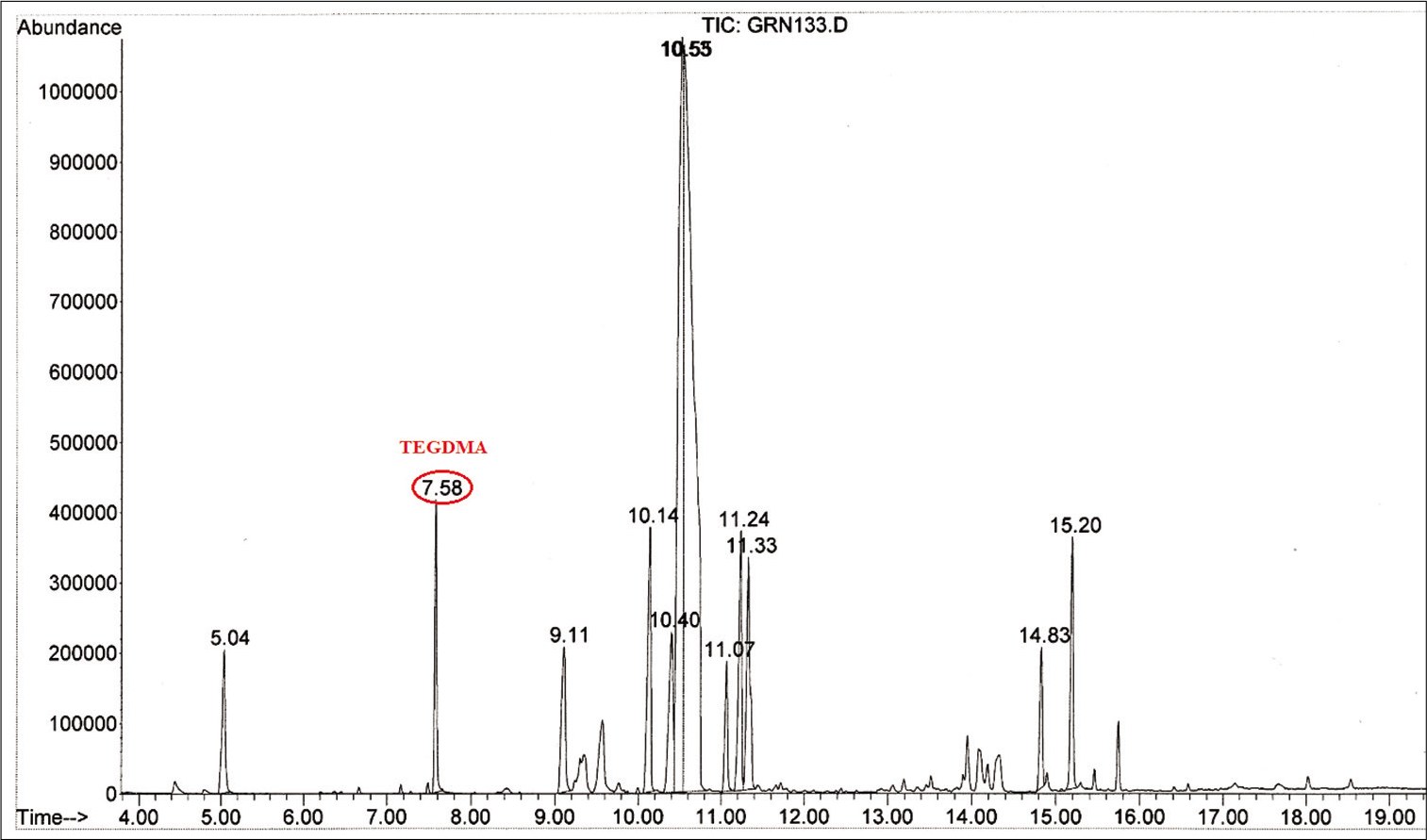
- Mass spectrum showing triethylene glycol dimethacrylate detected in the test group with Grengloo.
S. mutans has esterase activities at levels capable of degrading dental resin composites and adhesive system.[35] Consequently, the formation of bacteria dense biofilm can result in the ongoing destruction of the resin composite.
Many groups have studied the degradation of resin composites in the oral cavity. In the early 1990s, the focus of the studies shifted toward the chemical breakdown of these restorative materials because it was suggested that enzymes in the oral cavity may contribute to the degradation of resin composites.[41,42] Since then, a number of studies have investigated the degradation of resin composites in the presence of salivary-like enzymes[43-46] and the subsequent biological effects of the by-products on the surrounding bacteria and mammalian cells.[47-53] These biological processes that render commercial resins vulnerable to premature failure are currently beyond the control of the clinicians.
While there have been studies investigating the impact of composite degradation products on bacterial growth and virulence gene expression,[54,55] the potential effect of bacterial degradative activity on resin composites and adhesives has yet to be explored. Therefore, we hypothesized that, in addition to acid production, cariogenic bacteria contain esterase activities that degrade dental resin composites and adhesives.
The results of analyses of leachable substances (monomers, additives, and degradation products) from dental polymer-based materials may be influenced by the type of extraction media, the time and temperature of the extraction procedure, as well as the degree of curing and composition of the material.[39,56,57] It is known that methacrylates may degrade hydrolytically in aqueous environments.[39,58]
The results of this study support the hypothesis that cariogenic bacteria (S. mutans) contain esterase activities at levels capable of hydrolytic-mediated degradation of cured resin composites and adhesives.[35]
Orthodontic treatments increase the risk of the occurrence of carious lesions,[59] constituting a prejudice for patients and greatly compromising the success of these treatments. This risk is inherent both in the apparatus which causes an increase in plaque retention sites but also in a modification of the bacterial flora and in the age of patients.[60] The installation of orthodontic devices is followed by a modification in the oral ecosystem with an increase in the number of S. mutans and Lactobacilli.[23]
Human saliva has also been shown to hydrolyze resin composites and adhesives.[61]
Many bacterial species express esterases; however, the overall function of S. mutans esterases and, more specifically, their importance in contributing to the biodegradation process of dental resin composite restorations is not well-understood. In other bacteria, esterases have been linked to virulence and pathogenesis.[32,62,63]
CONCLUSION
S. mutans can hydrolyze long-term orthodontic composites
Monomers such as BPA and TEGDMA may be present in degradation products
Leaching compounds can be separate and identify by GC–MS technique.
Acknowledgments
The GC–MS analyses were performed to the Laboratoire National de Santé Publique of Ouagadougou.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Epoxy adhesives for orthodontic attachments: Progress report. Am J Orthod. 1965;51:901-12.
- [CrossRef] [Google Scholar]
- Release of bisphenol A and TEGDMA from orthodontic composite resins. IOSR J Dent Med Sci. 2019;18:73-7.
- [Google Scholar]
- New inorganic components for dental filling composites. Mon Chem. 2005;136:21-45.
- [CrossRef] [Google Scholar]
- Bisphenol a release from orthodontic adhesives measured in vitro and in vivo with gas chromatography. Am J Orthod Dentofacial Orthop. 2017;151:477-83.
- [CrossRef] [PubMed] [Google Scholar]
- Salivary bisphenol-a levels due to dental sealant/resin: A case-control study in Korean children. J Korean Med Sci. 2012;27:1098-104.
- [CrossRef] [PubMed] [Google Scholar]
- Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res. 1998;41:474-80.
- [CrossRef] [Google Scholar]
- Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res. 1991;70:1450-5.
- [CrossRef] [PubMed] [Google Scholar]
- Bisphenol-a content of resin monomers and related degradation products. Clin Oral Investig. 1999;3:114-9.
- [CrossRef] [PubMed] [Google Scholar]
- How much do resin-based dental materials release? A meta-analytical approach. Dent Mater. 2011;27:723-47.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178-98.
- [CrossRef] [PubMed] [Google Scholar]
- In vivo effects of bisphenol a in laboratory rodent studies. Reprod Toxicol. 2007;24:199-224.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of bisphenol a on adult male mouse fertility. Eur J Oral Sci. 2002;110:163-7.
- [CrossRef] [PubMed] [Google Scholar]
- Leached components from dental composites and their effects on fertility of female mice. Eur J Oral Sci. 2004;112:267-72.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization and in vitro estrogenicity of orthodontic adhesive particulates produced by simulated debonding. Dent Mater. 2009;25:376-82.
- [CrossRef] [PubMed] [Google Scholar]
- Bisphenol-a and residual monomer leaching from orthodontic adhesive resins and polycarbonate brackets: A systematic review. Am J Orthod Dentofacial Orthop. 2013;143(Suppl 1):104-12.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro elution of leachable components from dental sealants. J Am Dent Assoc. 1997;128:1517-23.
- [CrossRef] [PubMed] [Google Scholar]
- Glutathione level and genotoxicity in human oral keratinocytes exposed to TEGDMA. J Biomed Mater Res B Appl Biomater. 2012;100:391-9.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro estrogenicity of resin composites. J Dent Res. 2004;83:222-6.
- [CrossRef] [PubMed] [Google Scholar]
- Independent and combined cytotoxicity and genotoxicity of triethylene glycol dimethacrylate and urethane dimethacrylate. Mol Biol Rep. 2011;38:4603-11.
- [CrossRef] [PubMed] [Google Scholar]
- NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. Ntp Cerhr Mon. 2008;22:1-64.
- [Google Scholar]
- Detection of microecological phenomena in filled teeth, I. Phenomena in gab between restoration and cavity. Microb Ecol Health Dis. 1991;3:51-7.
- [CrossRef] [Google Scholar]
- Prediction of secondary caries around tooth colored restorations: A clinical and microbiological study. J Dent Res. 1996;75:1942-6.
- [CrossRef] [PubMed] [Google Scholar]
- Enamel demineralization in orthodontics, Rational use of fluoride in prevention and treatment. Rev Mens Suisse Odontostomatol. 2012;122:937-47.
- [Google Scholar]
- Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988;94:68-73.
- [CrossRef] [Google Scholar]
- Influences of orthodontic appliances on oral populations of mutans streptococci. Oral Microbiol Immunol. 2002;17:65-71.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of changes in Streptococcus mutans colonies in microflora of the Indian population with fixed orthodontics appliances. Dent Res J. 2016;13:309-14.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of orthodontic treatment on saliva, plaque and the levels of Streptococcus mutans and Lactobacillus. Med Oral Patol Oral Cir Bucal. 2010;15:e924-9.
- [CrossRef] [PubMed] [Google Scholar]
- Archwire ligation techniques, microbial colonization, and periodontal status in orthodontically treated patients. Angle Orthod. 2005;75:231-6.
- [Google Scholar]
- Salivary Streptococcus mutans levels in patients before, during, and after orthodontic treatment. Am J Orthod Dentofacial Orthop. 1991;100:35-7.
- [CrossRef] [Google Scholar]
- Recolonization of mutans steptococci on teeth with orthodontic appliances after antimicrobial therapy. Eur J Orthod. 2005;27:489-93.
- [CrossRef] [PubMed] [Google Scholar]
- Salivary microbial and nonmicrobial parameters in children with fixed orthodontic appliances. Angle Orthod. 2011;81:901-6.
- [CrossRef] [PubMed] [Google Scholar]
- The secreted esterase of Group A Streptococcus is important for invasive skin infection and dissemination in mice. Infect Immun. 2009;77:5225-32.
- [CrossRef] [PubMed] [Google Scholar]
- Biodegradation of composite resin with ester linkages: Identifying human salivary enzyme activity with a potential role in the esterolytic process. Dent Mater. 2014;30:848-60.
- [CrossRef] [PubMed] [Google Scholar]
- Biodegradation of resin composites and adhesives by oral bacteria and saliva: A rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater. 2014;30:16-32.
- [CrossRef] [PubMed] [Google Scholar]
- Cariogenic bacteria degrade dental resin composites and adhesives. J Dent Res. 2013;92:989-94.
- [CrossRef] [PubMed] [Google Scholar]
- Degradation in the dentin-composite interface subjected to multi-species biofilm challenges. Acta Biomater. 2014;10:375-83.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of various resin composite (Co)monomers and extracts on two caries-associated micro-organisms in vitro. J Dent Res. 1998;77:60-7.
- [CrossRef] [PubMed] [Google Scholar]
- 10993-12: 2012, Biological Evaluations of Medical Devices. In: Part 12: Sample Preparation and Reference Materials. Geneva, Switzerland: International Organization for Standardization; 2012.
- [Google Scholar]
- Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136-51.
- [CrossRef] [PubMed] [Google Scholar]
- Biodeterioration/biodegradation of polymeric medical devices in situ. Int Biodeterior Biodegradation. 1994;34:95-130.
- [CrossRef] [Google Scholar]
- Non-linear in vitro wear of posterior composites with time. Dent Mater. 1991;7:258-62.
- [CrossRef] [Google Scholar]
- Enzymatic degradation of BisGMA/TEGDMA-polymers causing decreased microhardness and greater wear in vitro. Scand J Dent Res. 1990;98:351-5.
- [CrossRef] [PubMed] [Google Scholar]
- Change in surface hardness of Bis-GMA/TEGDMA polymer due to enzymatic action. J Dent Res. 1992;71:1851-3.
- [CrossRef] [PubMed] [Google Scholar]
- Mutual influence of cholesterol esterase and pseudocholinesterase on the biodegradation of dental composites. Biomaterials. 2004;25:1787-93.
- [CrossRef] [PubMed] [Google Scholar]
- Biodegradation of a dental composite by esterases: Dependence on enzyme concentration and specificity. J Biomater Sci Polym Ed. 2003;14:837-49.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of resin chemistry on a dental composite's biodegradation. J Biomed Mater Res A. 2004;69:233-46.
- [CrossRef] [PubMed] [Google Scholar]
- Cell death effects of resin-based dental material compounds and mercurial in human gingival fibroblasts. Arch Toxicol. 2006;80:370-7.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of Bis-GMA on cyclooxygenase-2 expression, PGE2 production and cytotoxicity via reactive oxygen species-and MEK/ERK-dependent and independent pathways. Biomaterials. 2009;30:4070-7.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of triethylene glycol derived from dental composite resins on the regulation of Streptococcus mutans gene expression. Biomaterials. 2009;30:452-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cytotoxicity of resin monomers on human gingival fibroblasts and HaCaT keratinocytes. Dent Mater. 2007;23:40-4.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of light-curing time on the cytotoxicity of a restorative composite resin on odontoblast-like cells. J Appl Oral Sci. 2010;18:461-6.
- [CrossRef] [PubMed] [Google Scholar]
- Carboxylesterase expression in human dental pulp cells: Role in regulation of BisGMA-induced prostanoid production and cytotoxicity. Acta Biomater. 2012;8:1380-7.
- [CrossRef] [PubMed] [Google Scholar]
- Intracellular glutathione: A main factor in TEGDMA-induced cytotoxicity? Dent Mater. 2012;28:442-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of composite resin biodegradation products on oral streptococcal growth. Biomaterials. 2004;25:5467-72.
- [CrossRef] [PubMed] [Google Scholar]
- Composite resin degradation product from BisGMA monomer modulate the expression of genes associated with biofilm formation and other virulence factors in Streptococcus mutans. J Biomed Mater Res A. 2009;88:551-60.
- [CrossRef] [PubMed] [Google Scholar]
- The release of residual monomeric methyl methacrylate from acrylic appliances in the human mouth: An assay for monomer in saliva. J Dent Res. 1988;67:1295-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hydrolytic stability of self-etching adhesive systems. J Adhes Dent. 2005;7:107-16.
- [Google Scholar]
- Effect of polymerization temperature and time on the residual monomer content of denture base polymers. Eur J Oral Sci. 1998;106:588-93.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of caries in orthodontics: Descriptive study on 155 patients. Rev Stomatol Chir Maxillofac. 2010;111:276-9.
- [CrossRef] [PubMed] [Google Scholar]
- Caries risk and orthodontic treatment. Int Orthod. 2010;8:28-45.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of salivary esterase on the integrity and fracture toughness of the dentin-resin interface. J Biomed Mater Res B Appl Biomater. 2010;94:230-7.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of a novel cell wall-anchored protein with carboxylesterase activity required for virulence in Mycobacterium tuberculosis. J Biol Chem. 2007;282:18348-56.
- [CrossRef] [PubMed] [Google Scholar]
- The early response to acid shock in Lactobacillus reuteri involves the ClpL chaperone and a putative cell wall-altering esterase. Appl Environ Microbiol. 2007;73:3924-35.
- [CrossRef] [PubMed] [Google Scholar]






