Translate this page into:
Levels of matrix metalloproteinase-7 and osteopontin in human gingival crevicular fluid during initial tooth movement
Address for Correspondence: Dr. Amol Patil, Department of Orthodontics and Dentofacial Orthopedics, Dental College and Hospital, Bharati Vidyapeeth Deemed University, Pune, Maharashtra, India. E-mail: amolp66@yahoo.com
This article was originally published by Wolters Kluwer and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Purpose
During orthodontic treatment, the early response of periodontal tissues to mechanical stress involves several metabolic changes that allow tooth movement. The purpose of this investigation was to evaluate osteopontin (OPN) and matrix metalloproteinase (MMP)-7 in the gingival crevicular fluid (GCF) of human teeth exposed to orthodontic force.
Materials and Methods
GCF samples were obtained from 15 healthy orthodontic patients (age, 12-22 years). In each patient, the left maxillary canine having the fixed orthodontic appliance was used as the test tooth, and its antagonist, with no appliance, was the control. Orthodontic force, 75 g was applied using a 16 × 22 beta titanium closing loop. The GCF sampling on the disto-buccal aspects of experimental and control tooth was performed at specific time interval with sterilized absorbent paper point. Processing was carried out with enzyme-linked immunosorbent assay to detect OPN and MMP-7 levels.
Results
The peak level of OPN was seen after 1 h application of orthodontic force which was 1280.36 pg/ml ± 185.02. The peak level of MMP-7 was seen at 0 h which was 598.3 pg/ml ± 107.5. The levels of OPN after 1 h increased to 1280.36 pg/ml ± 185.02, and they decreased at 24 h to 1012.86 pg/ml ± 168.47 (P = 0.001). The levels of MMP-7 after 1 h decreased to 478 pg/ml ± 99.7 which increased at 24 h to 526.9 pg/ml ± 99.2.
Conclusions
Orthodontic forces affect both OPN and MMP-7 protein levels on the compression side in a time-dependent fashion.
Keywords
Compression side
enzyme-linked immunosorbent assay
gingival crevicular fluid
matrix metalloproteinase-7
osteopontin
orthodontic tooth movement
INTRODUCTION
During orthodontic tooth movement, structural and biochemical changes occur in the periodontal ligament (PDL) space.[1,2] Analysis of various cell mediators, enzymes, markers in the gingival crevicular fluid (GCF) has been performed for better understanding of the biological responses to orthodontic force. Several cell mediators, such as interleukin 1 β (IL1-β), IL6, β2 microglobulin, tumor necrosis factor-α (TNF-α),[3] prostaglandin E,[4] alkaline phosphatase, and aspartate aminotransferase[5] are found to be significantly elevated in GCF of a tooth to which orthodontic force was applied. Thus, further research of these biochemical mediators in GCF and their mechanisms will provide better and effective understanding about tooth movement.
Matrix metalloproteinases (MMPs) are enzymes that play a central role in PDL remodeling. MMPs are a family of zinc-dependent endopeptidases comprising more than 25 enzymes that regulate many biological processes. Based on primary structure, substrate specificity, and cell localization, these enzymes can be divided into at least four main subfamilies, including collagenases and gelatinases. Because of the properties of disintegrating native fibrillar collagen by MMP, some studies focus[6-9] on the in vivo and in vitro levels of various MMPs in the gingivae, gingival and periodontal fibroblasts, and GCF in response to orthodontic forces and periodontal diseases. MMP-7 also known as matrilysin has been shown to be associated with the degradation of various noncollagenous extracellular matrix (ECM) and basement membrane (BM) components, and to be capable of activating several other latent collagenolytic MMPs.[10] Role of MMP-7 has never been analyzed for orthodontic purpose.
Osteopontin (OPN) is a major noncollagenous bone matrix protein. A sialic acid — rich phosphorylated glycoprotein originally isolated from the bone, it contains a motif of glycine-arginine-glycine-aspartic acid-serine amino acid sequence that promotes cell attachment via cellular αvb3 integrin.[11] OPN is thought to promote or regulate the adhesion, attachment, and spreading of osteoclasts to the bone surface during bone resorption.[12] It is known to be produced by osteoblasts[13] as well as osteoclasts[14] and is considered to play important roles in bone formation, resorption, and remodeling.[15] Only physiological tooth movement, OPN mRNA was expressed in osteoclasts and osteocytes only in the resorption site of the septum. These findings strongly suggest that OPN participates in bone resorption. Denhardt and Guo[15] (1993) have shown that MMP-7 has a cleaving effect on OPN, which acts on the amino acid chains of the OPN structure leading to the reduction of OPN.
In this study, the presence and levels of MMP-7 and OPN in GCF following application of orthodontic force were analyzed on the compression side as levels of these have never been analyzed for orthodontic purpose.
MATERIALS AND METHODS
Fifteen subjects, eight males and seven females, between the ages from 12 to 22 years were selected for the study. Following were the inclusion criteria; subjects with:
Good health,
Healthy periodontium (color of gingiva, absence of bleeding on probing and clinical appearance of the gingiva were the only criteria used to evaluate normal gingival). No other criteria/index was used,
Requiring orthodontic treatment with extractions of either maxillary or mandibular first premolars.
Following were the exclusion criteria; subjects:
With systemic disorders,
On nonsteroidal anti-inflammatory drugs (NSAID’s) therapy for any reason. The other drugs were also considered as patients were asked about medication for any systemic diseases. NSAIDs were mentioned specifically because they are not necessarily used for only systemic diseases,
With calcium metabolism disorders,
With evidence of periodontal/gingival pathology and/ or gingival recession in canine region.
The study was approved by the scientific and Ethical Committee of the university.
As per the orthodontic treatment plan, all first premolars were extracted. One week after extractions of premolars the molars were banded and canine was bonded with preadjusted edgewise appliance (3M Unitek 0.018”). In upper left quadrant 16 × 22 beta titanium sectional tear drop closing loop was fabricated and placed from left canine to left first molar bypassing the premolar and the closing loop was activated as shown in Figure 1. The force exerted was 75 g which was measured with the help of a dontrix tension gauge.
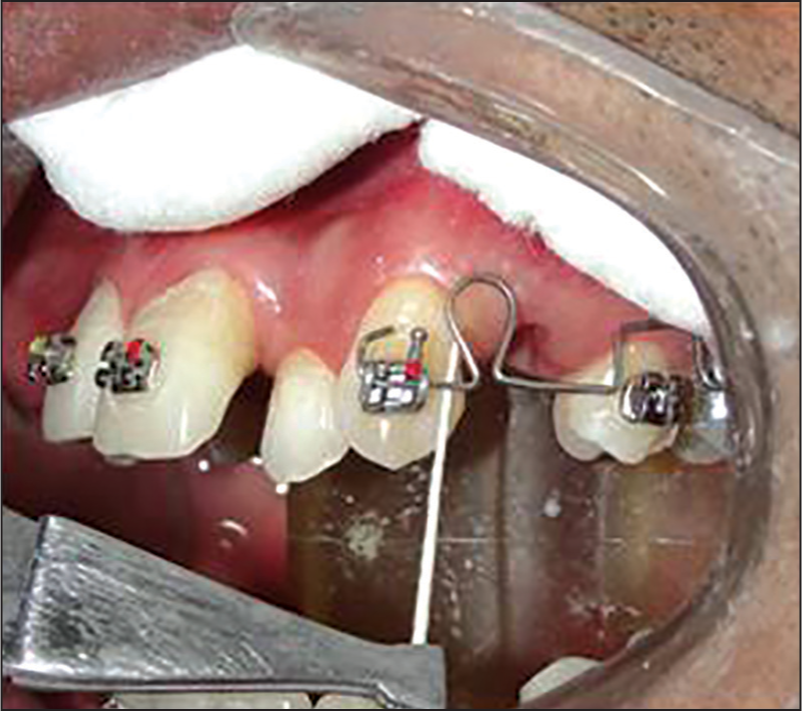
- Sample collection with the help of sterilized absorbent paper point International Organization for Standardization 40
The GCF was collected as per Burke et al. (2002).[16] Each sample was immediately transferred into microcentrifuge tube containing 500 μL of phosphate-buffered saline (PBS-A) and stored at −20°C. Subsequent samples were collected at 0, 1 and 24 h, respectively, at test and control side as tabulated in Table 1.
| Test number | Duration | Right side control | Left side test |
|---|---|---|---|
| TO | Oh | CTO | — |
| T1 | 1-h | CT1 | TT1 |
| T2 | 24 h | CT2 | TT2 |
Determination of osteopontin and matrix metalloproteinase-7 levels in gingival crevicular fluid
Human total MMP-7 Quantikine enzyme-linked immunosorbent assay (ELISA) kit and Human OPN Quantikine ELISA (R & D Systems, 2010, USA) were used. The samples were thawed to room temperature. The samples were centrifuged at 2500 rpm for 20 min at 4°C, to elute the cellular material in gingival crevicular fluid which is absorbed on absorbent paper points into PBS-A. A standard graph for the ELISA kit was prepared to get the results in picogram/milliliter (pg/ml). The prepared standard graphs had absorbance values on the Y-axis and the corresponding concentration of OPN [Figure 2] and MMP-7 [Figure 3] in pg/ml on the X-axis.
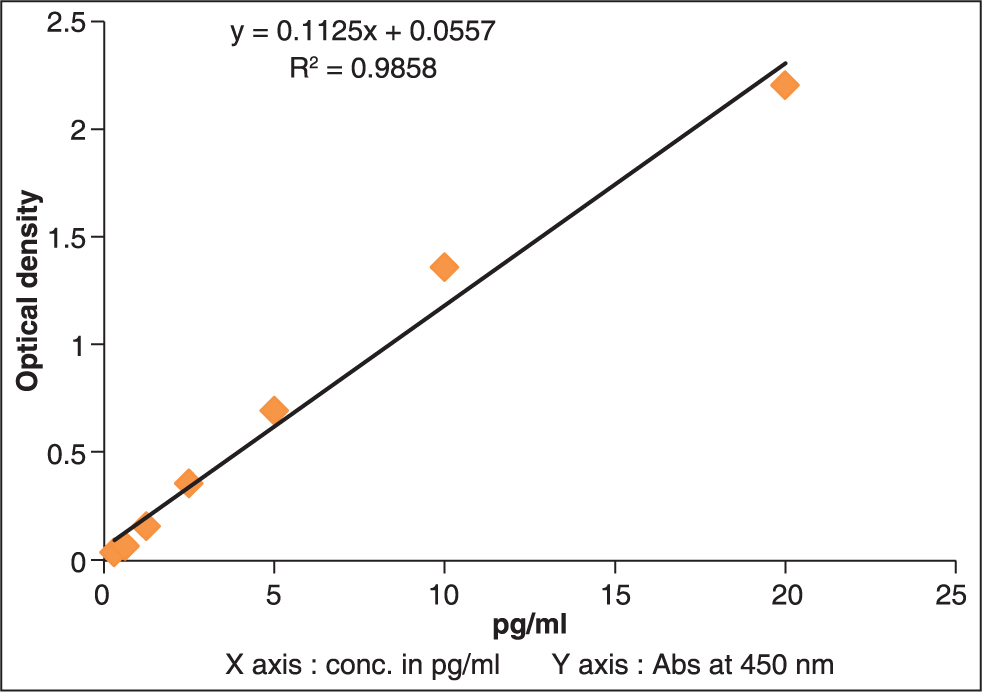
- Standard graph for osteopontin
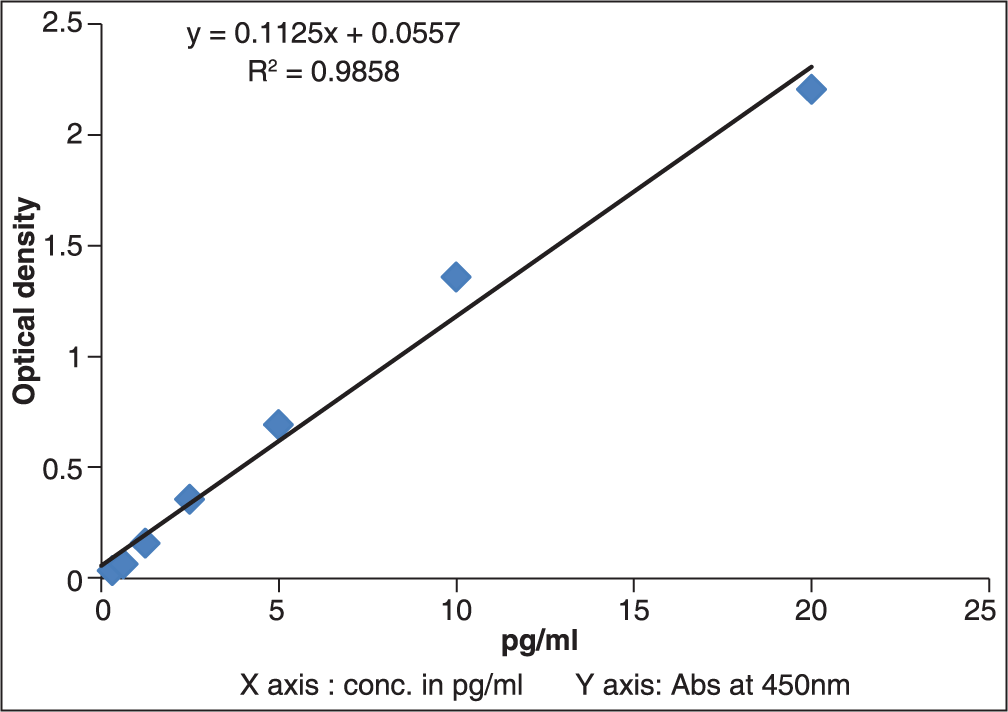
- Standard graph for matrix metalloproteinase-7
The concentration of OPN and MMP-7 was determined using a sandwich type human OPN and MMP-7 ELISA kit (Quantikine Assay Designs, USA). 100 μL of assay diluent RD1-6 was added to each well for OPN and RD1-52 for MMP-7. It was mixed well before and during use. Then 50 μL of sample was added per well. It was covered with an adhesive strip provided and incubated for 2 h at room temperature. Then each well was aspirated and washed with wash buffer (400 μL), repeating the process three times for a total of four washes. Then 200 μL of OPN conjugate for OPN and MMP-7 conjugate for MMP-7 was added to each well and covered with a new adhesive strip and incubated for 2 h at room temperature. The aspiration/ wash was carried out again. Then 200 μL of substrate solution was added to each well and incubated for 30 min at room temperature. It was protected from light. Lastly, 50 μL of stop solution was added to each well, and the plate was read immediately for absorbance of each well using a microplate (ELISA) reader set at a wavelength of 450 nm. The concentration of OPN and MMP-7 in the tested samples was calculated using the standard curve plotted with the optical density values obtained from the assay. This test resulted into yellow color and blue color for the sample which were positive for OPN and MMP-7, respectively. The results were tabulated, and the absorbance readings were used to get the exact concentration of OPN and MMP-7 using the standard graph prepared.
Statistical analysis
The entire data were analyzed using Statistical Package for Social Sciences (IBM SPSS Version 11.5, Java Platform, USA) for MS Windows. The data on OPN and MMP-7 are shown using mean ± standard deviation. The significance of difference of mean values for OPN and MMP-7 between the two study groups (test group compared with control group) was tested using Mann-Whitney U-test (nonparametric test for comparing two independent groups). In order to test the statistical significance of OPN and MMP-7 within group, Wilcoxon’s Signed Rank test (nonparametric test for comparing two related groups) was used. P < 0.05 were considered statistically significant. All the hypotheses were formulated using two-tailed alternatives against each null hypothesis.
RESULTS
Results of the study are summarized in Tables 2 and 3.
| Test duration | Test number | Right side control group | Test number | Left side test group | P | |||
|---|---|---|---|---|---|---|---|---|
| Mean (pg/ml) | SD | Mean (pg/ml) | SD | |||||
| 0 h (TO) | CTO | 732.6 | 88.9 | — | — | — | ||
| 1-h (T1) | CT1 | 736.0 | 101.3 | TT1 | 1280.36 | 185.02 | 0.001 | |
| 24 h (T2) | CT2 | 806.2 | 104.7 | TT2 | 1012.86 | 168.47 | 0.001 | |
SD – Standard deviation; OPN – Osteopontin
| Test duration | Test number | Right side control group | Test number | Left side test group | P | |||
|---|---|---|---|---|---|---|---|---|
| Mean (pg/ml) | SD | Mean (pg/ml) | SD | |||||
| 0 h (TO) | CTO | 598.3 | 107.5 | — | — | — | — | |
| 1-h (T1) | CT1 | 595.9 | 111.7 | TT1 | 478.0 | 99.7 | 0.001 | |
| 24 h (T2) | CT2 | 552.8 | 111.9 | TT2 | 526.9 | 99.2 | 0.285 | |
MMP – Matrix metalloproteinase; SD – Standard deviation
Osteopontin
The mean value of OPN at TT1 (1280.36 pg/ml ± 185.02) is increased (P < 0.001) as compared to CT1 (736.0 pg/ ml ± 101.3) indicating increased level of OPN in GCF after application of orthodontic force for 1 h. Similarly, mean value of OPN at TT2 (1012.86 pg/ml ± 168.47) is increased (P < 0.001) as compared to CT2 (806.2 pg/ml ± 104.7) indicating significantly higher level of OPN in GCF after 24 h. The above result is summarized in Table 2 and diagrammatically represented in Figure 4.
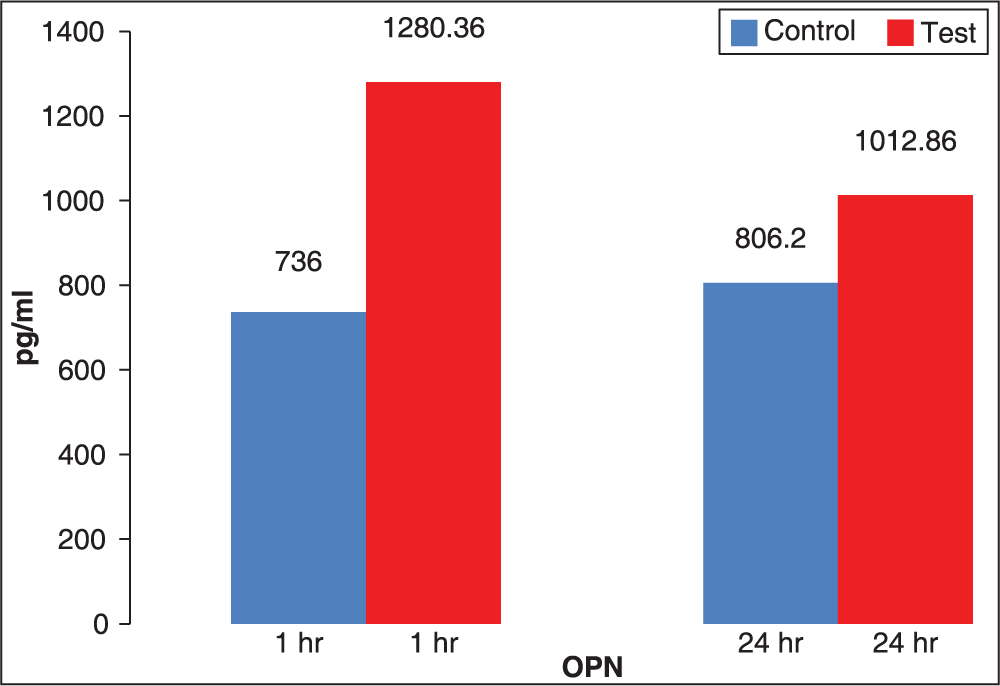
- Comparison of mean values of osteopontin expression in gingival crevicular fluid at 1 and 24 h
Matrix metalloproteinase-7
The mean value of MMP-7 at TT1 (478 pg/ml ± 99.7) is decreased as compared to CT1 (595.9 pg/ml ± 111.7) indicating decreased level of MMP-7 in GCF after 1 h application of orthodontic force (P = 0.001). Similarly, mean value of MMP-7 at TT2 (526.9 pg/ml ± 99.2) is decreased as compared to CT2 (552.8 pg/ml ± 111.9) (P = 0.285). The results of MMP-7 have been tabulated in Table 3 and diagrammatically represented in Figure 5.
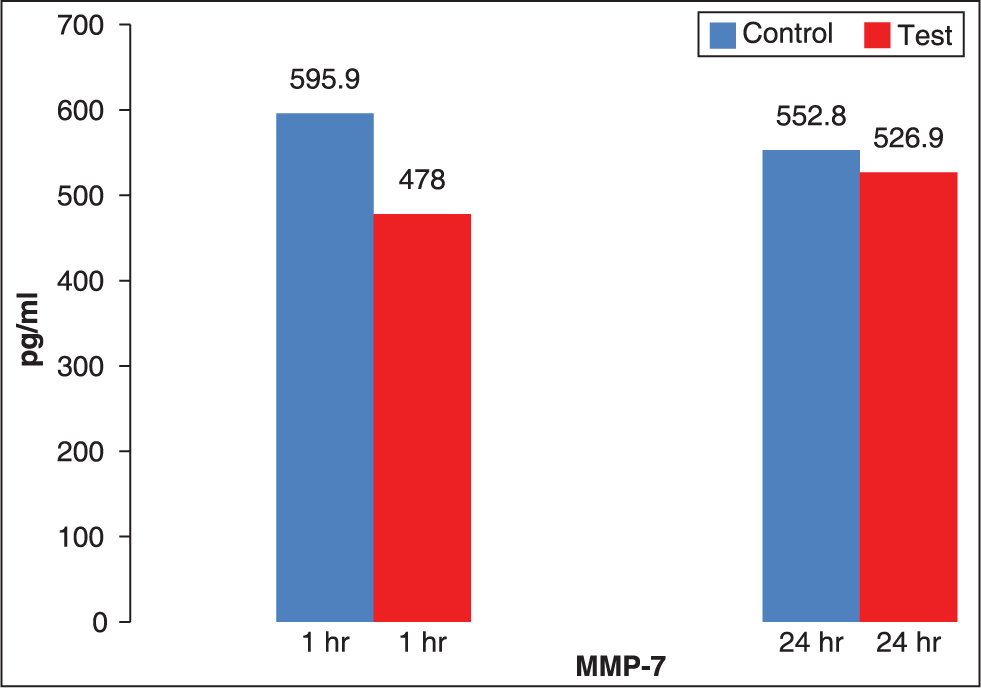
- Comparison of mean values of matrix metalloproteinase-7 expression in gingival crevicular fluid at 1 and 24 h
Thus the results can be summarized as follows as shown in Figure 4
The peak level of OPN was seen after 1 h application of orthodontic force which was 1280.36 pg/ml ± 185.02.
The level of MMP-7 was seen at 0 h that is before the application of orthodontic force which was 598.3 pg/ml ± 107.5.
The levels of OPN in the GCF after 1 h application of orthodontic force increased to 1280.36 pg/ml ± 185.02, and they decreased at 24 h to 1012.86 pg/ml ± 168.47 (P = 0.001).
The levels of MMP-7 in the GCF after 1 h application of orthodontic force decreased to 478 pg/ml ± 99.7 which increased at 24 h to 526.9 pg/ml ± 99.2 close to the control values.
DISCUSSION
Previous research[3-9] has suggested that local mediators such as prostaglandins, ILs and growth factors play an important role in bone remodeling induced by orthodontic forces. The levels of these mediators in GCF have been well demonstrated to be responsive to orthodontic force in humans. However, there have been no studies focused on the OPN and MMP-7 dependent remodeling of human periodontal tissues during orthodontic tooth movement. To allow orthodontic tooth movement, there must be a delicate and well-balanced ECM remodeling process in the periodontum while maintaining periodontal functional integrity. It is thought that increased or decreased expression of specific m-RNA and production of proteins by PDL cells are significant parts of their adaptive responses to several types of mechanical stress.
Gingival crevicular fluid a site for markers
Analysis of GCF samples might be a good way to examine the ongoing biochemical processes associated with bone turnover during orthodontic tooth movement. Tissue samples of the PDL or the bone undergoing resorption would provide a more direct site for measuring changes in the periodontium, but cannot be obtained from human subjects. GCF provides an indirect measurement of changes occurring in the PDL. Thus, GCF is a promising noninvasive method for determining tissue changes in the periodontium as the enzymes in the periodontium are known to diffuse into it.[3-9] Estimation of markers, enzymes gives insight in understanding the changes occurring in periodontium during orthodontic tooth movement. GCF has been used to determine various enzymes like IL1-β, IL6, TNF-α, prostaglandin E, alkaline phosphatase and aspartate aminotransferase, β2 microglobulin, cystatin, cathepsin B, MMP, and osteoprotegerin.
Matrix metalloproteinase-7
Matrix metalloproteinases, which have been implicated in the remodeling of bone and connective tissues, and also seem to be involved in PDL remodeling during orthodontic movement. This has been proved by decreased orthodontic tooth movement in mice due to administration of MMP inhibitors.[17] MMP-7 has been shown to be associated with the degradation of various noncollagenous ECM and BM components, and to be capable of activating several other latent collagenolytic MMPs, including MMP-8.[18]
Osteopontin
Mechanical forces have been suggested to act at the sites of cell attachment, possibly involving ECM proteins including OPN, to generate a shear stress at adhesion plaques, to transmit signals via integrins to the cytoskeleton, and to modify cell shape and gene expression.[19] It is known that mechanical stress induces OPN production, and this phenomenon has been suggested to trigger events in mechanical stress — dependent bone formation and bone resorption. The presence of OPN is critical in mediating the effect of physical force to change the metabolism of bone. With regard to unloading-induced bone resorption, OPN could be required to modulate the development or recruitment of osteoclast precursors upon unloading to augment the number of osteoclasts and promote bone resorption, even though not required for baseline of osteoclastogenesis. OPN could act as a chemoattractant as observed in other types of cells.[20] Thus, the role of OPN would be to provide critical regulatory information for the recruitment and/or development of osteoclasts in a situation of acute bone resorption in vivo and to transmit the signals to the cytoskeleton. Thus, the results in the study showed an increased level of OPN, which can be attributed to the enhanced remodeling process in the first few hours.
Co-relation of matrix metalloproteinase-7 and osteopontin
Results have shown that when there is an increase in the levels of OPN after 1st h of application of orthodontic force there is a decrease in the levels of MMP-7 after the 1st h of application of orthodontic force. Furthermore, the results show there is a decrease in levels of OPN after 24 h of application of force there is an increase in the levels of MMP-7. From this we can infer that the reduction of MMP-7 after the 1st h of application of orthodontic force allows OPN to increase, which is believed to regulate the release, attachment, and spreading of osteoclasts to the surface of bone. The increase of MMP-7 after the 24 h can be correlated with the decrease of OPN due to the cleaving effect of MMP-7 on OPN which acts on the amino acid chains of the OPN structure leading to the reduction of OPN in the GCF after the first 24 h (Denhardt and Guo[15] 1993).We can, therefore, conclude that MMP-7 and OPN play a viable role to regulate the tooth movement [Figure 6].
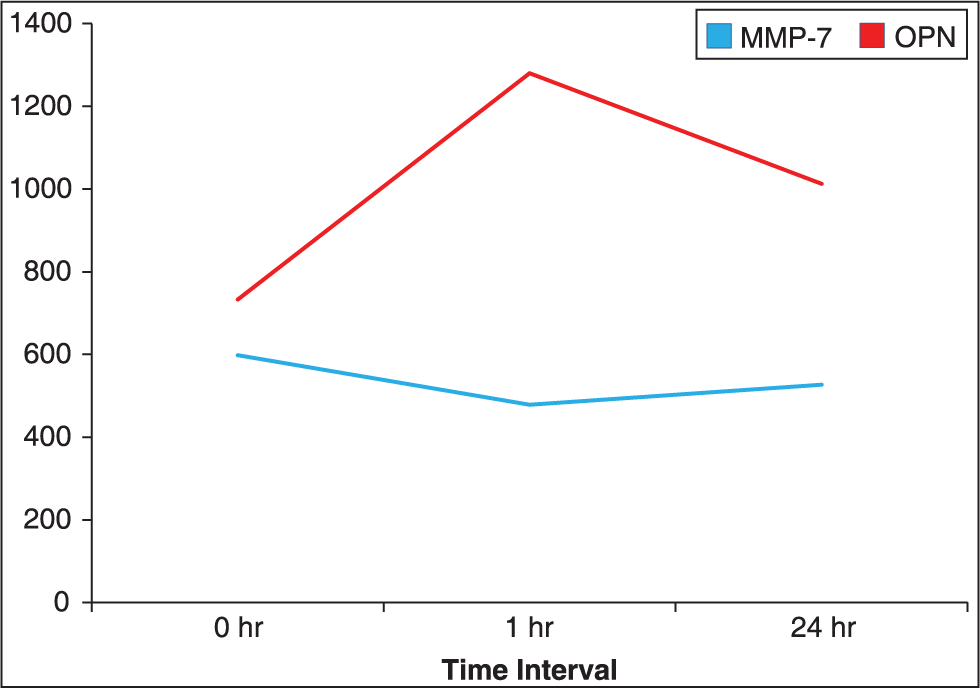
- Co-relation of matrix metalloproteinase-7 and osteopontin expression
Our results demonstrate for the first time in vivo that orthodontic forces modulate MMP-7 and OPN expression levels on the compression side and that this is a time-dependent regulation of their expression at the protein level. These conclusions, however, should be viewed cautiously, since the possibility of contamination between the two zones, that is, compression and tension zones in the gingival crevice could not be excluded, apart from the fact that the effects of force application (i.e., tension or compression), even if present, can never be totally predicted using this type of mechanical system.
CONCLUSIONS
Osteopontin and MMP-7 are detectable during initial tooth movement, and their respective immunoreactivity is assessable by the ELISA test technique in the GCF of experimental teeth.
Orthodontic forces affect both OPN and MMP-7 levels on the compression sides. OPN and MMP-7 expression vary in a time-dependent fashion.
References
- Expression of the type I collagen gene in rat periodontal ligament during tooth movement as revealed by in situ hybridization. Arch Oral Biol. 1994;39:289-94.
- [Google Scholar]
- Temporal and spatial expressions of type XII collagen in the remodeling periodontal ligament during experimental tooth movement. J Dent Res. 1995;74:313-8.
- [Google Scholar]
- Interleukin (IL)-1 beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta 2-microglobulin levels are elevated in gingival crevicular fluid during human orthodontic tooth movement. J Dent Res. 1996;75:562-7.
- [Google Scholar]
- Prostaglandin E (PGE) and interleukin-1 beta (IL-1 beta) levels in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1994;105:369-74.
- [Google Scholar]
- Aspartate aminotransferase activity in gingival crevicular fluid during orthodontic treatment. A controlled short-term longitudinal study. J Periodontol. 2003;74:145-52.
- [Google Scholar]
- Gingival crevicular fluid matrix metalloproteinase-25 and -26 levels in periodontal disease. J Periodontol. 2006;77:664-71.
- [Google Scholar]
- Levels of matrix metalloproteinases 1 and 2 in human gingival crevicular fluid during initial tooth movement. Am J Orthod Dentofacial Orthop. 2006;130:568.e11-6.
- [Google Scholar]
- The in vivo levels of matrix metalloproteinase-1 and -8 in gingival crevicular fluid during initial orthodontic tooth movement. J Dent Res. 2003;82:1018-22.
- [Google Scholar]
- Matrix metalloproteinase -2, -8, -9, and -13 in gingival crevicular fluid of short root anomaly patients. Eur J Orthod. 2003;25:365-9.
- [Google Scholar]
- Matrilysin (matrix metalloproteinase-7) expression in human junctional epithelium. J Dent Res. 2002;81:241-6.
- [Google Scholar]
- Osteopontin expression and function: Role in bone remodeling. J Cell Biochem Suppl. 1998;30-31:92-102.
- [Google Scholar]
- Osteopontin — a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990;87:4473-5.
- [Google Scholar]
- Expression of osteopontin mRNA by osteoclasts and osteoblasts in modelling adult human bone. J Cell Sci. 1993;104:1013-20.
- [Google Scholar]
- Identification of osteopontin in isolated rabbit osteoclasts. Biochem Biophys Res Commun. 1992;186:911-7.
- [Google Scholar]
- Expression of secretory proteins in oral fluid after orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2002;121:310-5.
- [Google Scholar]
- Collagenase 2 (MMP-8) expression in murine tissue-remodeling processes. Analysis of its potential role in postpartum involution of the uterus. J Biol Chem. 1998;273:23959-68.
- [Google Scholar]
- Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57:344-58.
- [Google Scholar]
- Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468-78.
- [Google Scholar]






