Translate this page into:
Molar extraction alters gastric mucosa and ghrelin expression in rat stomach: A preliminary study

*Corresponding author: Ippei Watari, Department of Orthodontic Science, Tokyo Medical and Dental University (TMDU), Tokyo, Japan. ippeiwatari@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Shams SM, Watari I, Saito E, Ono T. Molar extraction alters gastric mucosa and ghrelin expression in rat stomach: A preliminary study. APOS Trends Orthod 2022;12:86-93.
Abstract
Objectives:
Ghrelin is a key regulator of food intake and is considered a hunger hormone that affects cognition, memory, glucose metabolism, and antidepressant effects. Altered occlusion, such as a loss of molars, has been thought to retard digestive function. However, the association between occlusion and digestive function remains poorly understood. Here, we aimed to explore the effect of bilateral maxillary molar extraction on the gastrointestinal mucosa of growing rats and the expression of ghrelin and its receptor, growth hormone secretagogue receptor (GHSR).
Material and Methods:
Twenty-four male 5-week-old Wistar rats were divided into control (CON) and experimental (EXP) groups (n = 12/group). The rats in the EXP group underwent extraction of the bilateral maxillary first, second, and third molars under general anesthesia. Rats in the CON group underwent a sham operation. All rats in both the CON and EXP groups were fed a powder diet and water ad libitum. The body weight of all rats was monitored throughout the EXP period. Rats in both the CON and EXP groups were euthanized on days 14 and 28, and the stomachs were isolated and subjected to histological analysis. Paraffin serial sections were prepared using a microtome for hematoxylin-eosin and immunohistochemical staining using anti-ghrelin and anti-GHSR antibodies. The distribution and expression of ghrelin-immunopositive and GHSR cells were detected and observed under a light microscope. Data were statistically analyzed using t-tests (P < 0.05).
Results:
There were no significant differences in body weight between the CON and EXP groups throughout the EXP period. Histological analysis showed that the area of the submucosa (ASM), and the number of ghrelinimmunopositive cells were significantly decreased in the EXP group compared with the CON group on day 14. Alternatively, there was no significant difference in the ASM and the number of ghrelin-immunopositive cells between the CON and EXP groups on day 28, whereas the number of ghrelin receptors showed no differences across groups. Furthermore, the number of eosinophilic blood cells significantly increased in the EXP group on days 14 and 28.
Conclusion:
Our findings suggest that bilateral maxillary molar extraction may trigger stomach mucosal changes and alter digestive function through ghrelin expression in rats. This is the first report that occlusal deficiency could alter ghrelin expression in the mucosa of the rat stomach, thus raising concerns about the consequential role of ghrelin.
Keywords
Molar extraction
Digestive function
Ghrelin
GHSR
Stomach
Occlusion
INTRODUCTION
Ghrelin, a novel gastrointestinal hormone synthesized from the stomach mucosa is predominantly responsible for food intake and is considered a hunger hormone.[1] It has various functions in growth hormone release, glucose metabolism, and memory.[2] Ghrelin is a brain-gut peptide consisting of 28 amino acid chains, in which n-octanoylated is the third serine residue, and this side chain is required as a mechanism for ghrelin release activity. Ghrelin sequesters as an endogenous ligand for growth hormone secretagogue receptor (GHSR), also known as ghrelin receptor,[3] and regulates growth hormone release mechanism, manifesting from its regulation by hypothalamic growth-hormone-releasing hormone.[4] Apart from the two amino acids, human ghrelin is analogous to rat ghrelin, both of which manifest that the release of growth hormone from the pituitary may be regulated similar to hypothalamic growth-hormone-releasing hormone.[4] Parallel to the release of growth hormone, GHSR is important for appetite increase and energy storage, insulin secretion, gastric acid production and protection, gastrointestinal motility, cell proliferation, and death.[5]
As the first phase of the digestive mechanism, mastication plays the role of breaking down the foods into smaller fragments to accelerate the enzymatic process in the course of the late stage of digestion.[6] Tooth loss is a major cause of impaired mastication.[7] People with masticatory impairment can manage with feeding by either altering their dietary habits or swallowing coarse fragments that potentially cause digestive problems.[7]
Molar extraction influences the periodontium, temporomandibular joint, and brain function in rats.[8] Although a correlation between mastication and digestion has been suggested, a few studies have assessed the effect of tooth extraction on the gastrointestinal tract of growing rats. As ghrelin is a critical factor that controls (CON) appetite and energy expenditure, well-balanced occlusion is thought to bring physiological digestive function as well as normal appetite regulation. Based on this, we aimed to explore the effect of bilateral maxillary molar extraction on the gastrointestinal mucosa of growing rats, with a focus on the histological expression of ghrelin and GHSR.
MATERIAL AND METHODS
Experimental (EXP) animals
Twenty-four male 5-week-old Wistar rats were distributed into the CON and EXP groups (n = 12). The rats in the EXP group underwent extraction of the bilateral maxillary first, second, and third molars (BMME) under general anesthesia. General anesthesia was induced by inhalation of 4% isoflurane (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and intraperitoneal injections of pentobarbital sodium (30.0 mg/kg body weight; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan). Rats in the CON group underwent a sham operation. Extraction was performed to prevent molar occlusal contact, thus implying masticatory deficiency. All rats were fed a powder diet (CE2, Clear Tokyo, Japan) and water ad libitum. The body weight of all rats was monitored throughout the EXP period. The rats in both groups were euthanized on days 14 and 28. The stomach was isolated and subjected to histological analysis immediately after euthanization. All procedures in this study were performed after ethical approval (authorization number: A2020-069A) from the institutional ethical committee and under the Animal Care Standards of Tokyo Medical and Dental University (TMDU).
Preparation of the stomach for histological analysis
The portion of the gastric body of the stomach (3 cm proximal to the pylorus) was obtained as a specimen from the rat gastrointestinal tract. The isolated stomach was immediately immersed in 4% paraformaldehyde (PBS; Mildform R; Wako Pure Chemical Industries, Osaka, Japan) at 4°C. After washing with PBS, the samples were embedded in paraffin according to a standard protocol.[9] Paraffin serial sections of 5-µm thickness were prepared using a microtome (HistoCore MULTICUT R, Leica, Germany) histological observation and were observed under a light microscope (Microphoto-FXA; Nikon, Tokyo, Japan) equipped with a digital camera (DXm1200; Nikon, Tokyo, Japan) as described below.
Hematoxylin-eosin (HE) staining and morphometric evaluation
Sections with a thickness of 5µm were deparaffinized with xylene and rehydrated in a graded ethanol series to analyze the morphometric measurements. The sections were then stained with HE and observed under a light microscope (Microphoto-FXA; Nikon, Tokyo, Japan) at ×40 and ×100 magnification. Digital images were captured using a digital camera (DXM1200; Nikon). We determined the main regions of the mucosa and measured the width of each layer. First, the base layer was determined by the area where the chief cells were densely populated. Second, the neck layer of the mucosa was determined by parietal cells and another neck epithelial mucous cell populations. Third, the gastric pits were identified by depressions in the stomach, which denote entrances to the tubular-shaped gastric glands and transport gastric cell secretions. We drew a line to measure the width of each layer after the determination. The obtained mean of five locations per sample for each tissue was analyzed in randomly selected microscopic fields using imaging software. In HE staining, the total area of the submucosa (ASM) in the transverse section was measured by selecting three 3 fields of 400 µm (horizontal) × 200 µm (vertical) = 80,000 µm2 containing densely populated cells and vessels. The number of eosinophilic blood cells in the submucosa and the mucosa was counted by determining the number of eosinophil-stained blood cells in three randomly-selected 3 fields of 350 µm (horizontal) × 150 µm (vertical) = 52,500 µm2. To obtain mean values, three records per sample for each tissue were analyzed in randomly selected microscopic fields. All measures were analyzed blindly using imaging software (ImageJ 1.44; MD, United States).
Immunohistochemistry
The expression of ghrelin-immunopositive cells was detected by immunohistochemistry using an anti-ghrelin antibody (ab217011, Abcam, Cambridge, MA, USA). Staining was performed using the ABC method. Immunohistochemical staining was performed as previously described.[10] Briefly, deparaffinization of all sections was performed with xylene and then rehydrated in a graded ethanol series. We used electric stove treatment with an unmasking solution (antigen unmasking solution; Vector Laboratories, Burlingame, CA, USA) for antigen activation. The solution was prewarmed at 100°C, and then the section was immersed for 20 min, followed by incubation at room temperature for 20 min. Endogenous peroxidase was blocked with peroxidase-blocking solution (Agilent Technologies, Santa Clara, CA, USA) for 10 min at room temperature. To prevent nonspecific binding of antibodies, we incubated the sections with TBS containing 1% bovine serum albumin for 30 min at room temperature. Subsequently, the sections were incubated overnight at 4°C with an anti-ghrelin antibody (ab217011, Abcam, Cambridge, MA, USA) diluted 1:150 in TBS containing 0.3% Triton X-100 and 0.1% bovine serum albumin, followed by incubation with a diluted secondary anti-goat immunoglobulin G antibody (VECTASTAIN ABC Staining Kit; Vector Laboratories, Burlingame, CA, USA) for 30 min at room temperature. After applying an avidinbiotin macromolecule for 30 min at room temperature and incubation with 3,3- diaminobenzidine (DAB) for 15 s, the sections were washed in PBS. The sections were then counterstained with hematoxylin, rinsed for 15 min under running tap water, dehydrated with an ethanol series, cleared in xylene, and mounted with Mount-Quick “Aqueous” (Cosmo Bio Inc., Tokyo, Japan). The number of ghrelinimmunopositive cells was counted based on immunopositive mucosal cell density in the direction from the base to the neck of the mucosa with fields of 500 µm (horizontal) × 300 µm (vertical)= 150,000 µm2 per section under 100x magnification using light microscopy (DS-Ri1; Nikon, Tokyo, Japan). Three sections from each rat were examined.
For GHSR detection, along with the procedures mentioned above, rabbit anti- GHSR (ab85104 Abcam, Cambridge, MA, USA) diluted 1:150 in TBS containing 0.3% Triton X-100 and 0.1% bovine serum albumin) was used as the primary antibody and incubated overnight at 4°C. The number of the GHSR immunopositive cells were counted based on immunopositive mucosal cells density at the direction from the base to the neck of the mucosa with the fields of 250 µm (horizontal) × 150 µm (vertical) per section under ×200 magnification using light microscopy (DS-Ri1; Nikon, Tokyo, Japan). Three sections from each rat were examined.
The procedures for image capturing identification and processing were standardized before the images were captured. The number of ghrelin-immunopositive cells and GHSR immunopositive cells in each scanned image of the respective cells was computed using a digital image analyzer in the ImageJ 1.44 software. The mean number of immunopositive cells was obtained.[3,11]
Statistical analysis
All results were evaluated as the mean ± standard deviation. Comparisons between the CON and EXP groups were performed using the Student’s t-test using statistical analysis software (R ver. 3.6.1, Vienna, Austria). Differences with values of P<0.05 were set as statistically significant.
RESULTS
Bodyweight
No significant difference in body weight was observed between the CON and EXP groups throughout the EXP period [Figure 1]. This indicated that there were no metabolic differences in the general condition between the two groups.
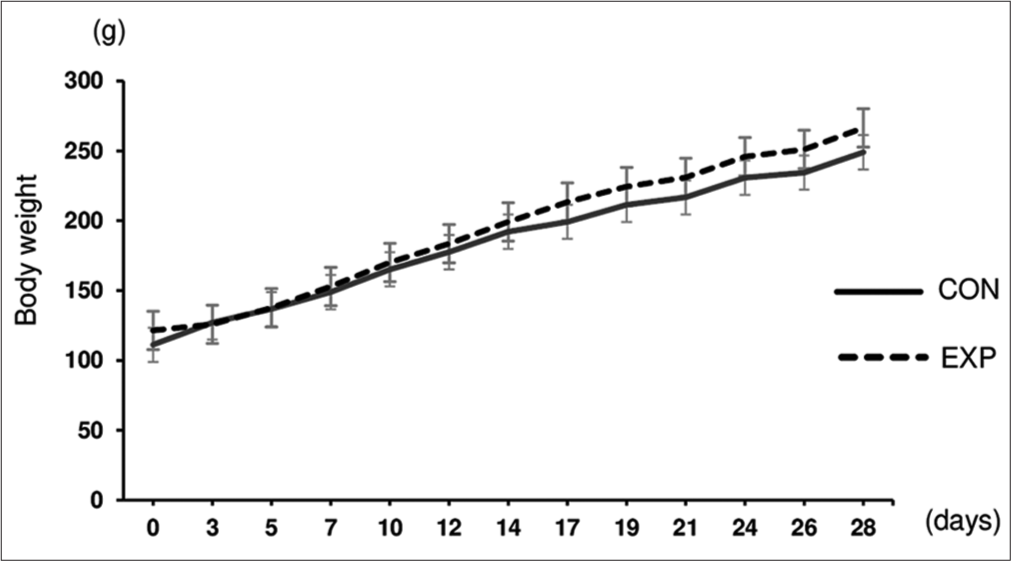
- Bodyweight of the rat. Changes in body weight during the experimental period in the CON and EXP groups (n = 12/group). Data are expressed as mean ± standard deviation.
Histological findings in HE staining
The cells and vessels were more distinguished on day 28 than on day 14 in both the CON and EXP groups in the transverse section of the stomach [Figure 2]. Moreover, the mucosal thickness appeared to be thinner in the EXP group than in the CON group on both days 14 and 28. There was a significant (P < 0.05) reduction in the width of the base [Figure 3b], neck [Figure 3c], and gastric pit [Figure 3d] of the mucosa in the EXP group compared with the CON group on both days 14 and 28. Compared with the CON group, hyperplasia of the cells was seen more densely in the EXP group on both days 14 and 28 [Figure 4A]. Morphometric analysis revealed that the ASM was significantly (P < 0.05) lower in the EXP group than in the CON group on day 14 [Figure 4B]. Alternatively, ASM showed no significant difference between the CON and EXP groups on day 28. The number of eosinophilic blood cells significantly (P < 0.05) increased in both the submucosa [Figure 4C] and mucosa [Figure 4D] in the EXP group when compared with the CON group.

- Hematoxylin-eosin staining in the transverse section of the stomach. Morphological observations in the CON (a and c) and EXP (b and d) groups. Bar = 100 µm. Magnification: ×40.

- Identification and measurement of the layers in the gastric body of the stomach (Hematoxylin-eosin staining). (a) The mucosa of the stomach consists of regions such as the Base, Neck, and Gastric pit. (b) Comparison of the width of the base of the mucosal layer between the CON and EXP groups (n = 6) in 14 and 28 days. (c) Comparison of the width of the neck of the mucosal layer between the CON and EXP groups (n = 6) in 14 and 28 days. (d) Comparison of the width of the gastric pit of the mucosal layer between the CON and EXP groups (n = 6) in 14 and 28 days. Data are expressed as the mean ± standard deviation (b-d). Bar = 100 µm. Magnification: ×100. *P < 0.05.

- Hematoxylin-eosin staining of a transverse section of the stomach. (A) Morphological observations in the CON (a and c) and EXP (b and d) groups. (B) Morphometric measurements of the submucosal area of the stomach. The areas were compared between the CON and EXP groups (n = 6) on days 14 and 28. (C) The relative number of eosinophilic blood cell count in the submucosa. The numbers in the EXP group were normalized to those in the CON group (n = 6). (D) The relative number of eosinophilic blood cell count in the mucosa. The numbers in the EXP group were normalized to those in the CON group (n = 6). Data are presented expressed as the mean ± standard deviation (B-D). Bar = 100 µm. Magnification: ×100. *P < 0.05.
Expression and distribution of ghrelin in the stomach
Ghrelin immunopositive cells were observed in the gastric body of the stomach [Figure 5A]. Ghrelin immunopositive cells were found in the mucosal layer. In the gastric body, most ghrelin-immunopositive cells were detected in the direction from the glandular base to the glandular neck region [Figure 5A]. The number of ghrelin-immunopositive cells was significantly lower in the EXP group than in the CON group on day 14.

- Immunohistochemical analysis of ghrelin expression. (A) Ghrelin was observed in the mucosa of the stomach in the CON (A and C) and EXP (b and d) groups. The rectangle indicates the area of measurement, whereas the arrow indicates ghrelin immunopositive cells. (B) The relative number of ghrelin immunopositive cells in the mucosa. The numbers in the EXP group were normalized to those in the CON group (n = 6). Data are presented expressed as the mean ± standard deviation (b). Bar = 100 µm. Magnification: ×100. *P < 0.05.
There were no significant differences in the number of ghrelin-immunopositive cells between the two groups on day 28 after BMME [Figure 5B].
GHSR in the gastric mucosa
We found GHSR immunopositive cells in the mucous area [Figure 6A]. There was no significant difference in the number of GHSR immunopositive cells between the CON and EXP groups on days 14 and 28 [Figure 6B].

- Immunohistochemical images of GHSR expression. (A) GHSR was observed in the mucosa of the stomach in the CON (a andc) and EXP (b and d) groups (n = 6). The rectangle indicates the area of measurement, whereas the arrow indicates the ghrelin receptor immunopositive cells. (b) The relative number of GHSR immunopositive cells in the mucosa. The numbers in the EXP group were normalized to those in the CON group (n = 6). Data are presented expressed as the mean ± standard deviation (B). Bar = 50 µm. Magnification: ×200. *P < 0.05.
DISCUSSION
Mastication plays a crucial role in the digestive process by pulverizing the foods into small particles and then, facilitating enzymatic processing during later stages of digestion. Here, we used a rat EXP model which underwent BMME to investigate the morphological changes in the gastric body and alterations in the expression and distribution of both ghrelin and GHSR in the stomach.
Tooth extraction has several effects on mastication, impairing masticatory function through oro-sensory signals. As a result, impaired mastication throughout the eating process reduces the cephalic phase responses of the digestion and delays the onset of satiety.[12] Therefore, it provides insight into changes in digestive function, including gastric body function and ghrelin production.
Changes in the mucosal layer were observed on days 14 and 28 after BMME. The widths of the three layers of the mucosa were reduced in the EXP group on days 14 and 28, which denote the changes in the surface. The reduction of gastric pit width in the EXP group revealed the indication of impaired communication between the lumen of the stomach and gastric pit, responsible for transporting gastric cell secretion. This suggests that the function of the glandular stomach in the enzymatic and hydrolytic digestion of ingested food substances was obstructed. However, it should be mentioned that the zone between the gastric pit and the neck region contains stem cells that are responsible for the renewal of the gastric mucosa with apoptosis.[13,14]
The cells and vessels were more distinguished on day 28 after BMME in terms of the respective CON and EXP groups in the transverse section of the tissue. ASM decreased in the EXP group by day 14. Eosinophilic blood cells were seen densely in the EXP group on days 14 and 28 after BMME. Blood cells are involved in the immune response to macrophages. In addition, hyperplasia of the cells was observed in the EXP group. In our study, the comparison of body weight between the CON and EXP animals indicated that there were no significant metabolic differences in general conditions. Following BMME (14 days), ASM significantly decreased in the EXP group. Hypotrophy and/or ulceration of the stomach layer is seen in other human tooth loss studies.[15] The inadequate masticatory function increases the gastric emptying rate,[16] suggesting a possible relationship between mastication and digestion in the stomach. Although a soft diet was commonly provided for both groups, chewing inability might have altered the submucosa layer in the EXP group, indicating the occurrence of a retarded effect on mucosa.
On day 28 after BMME, the submucosal layer showed a change with the increasing area, indicating the occurrence of defense and repair mechanisms, comparable to a previous study.[14] Any alteration in gastric layer healing is naturally programmed for the repair process. This process consists of a response to inflammation or any other changes, cell proliferation, re-epithelialization, formation of granulation tissue, angiogenesis, and scar formation.[14,17]
We observed that the number of eosinophilic blood cells increased significantly in the submucous and mucous layers of the stomach body in the EXP group after 14 and 28 days of tooth extraction, indicating that inflammation might have occurred throughout the EXP period in the EXP animals. Alterations in blood cells have been ascribed to inflammation.[18] Moreover, the natural repair process in rats requires an immune response through pluripotent hematopoiesis in the damaged area accompanied by macrophages.[14] Our findings could also explain the changes in the submucosal layer area.
Ghrelin immunopositive cells are observable in the mucosal layer of the stomach body, however, not in the myenteric plexus, due to higher density.[19] With pleomorphic changes in the stomach, the GHSR immunopositive cells showed a decrease on day 14 and an attenuated suppression on day 28 following BMME. These findings indicated alteration of the gastric layer and metabolism. The entire gastrointestinal tract undergoes a relentless renewal by cellular turnover. In this process, epithelial cells of the gastrointestinal tract contribute largely to cellular mass turnover.[20] Naturally, the integrity of the gastric mucosa is preserved in mammals with three defense mechanisms: preepithelial (mucus–bicarbonate–phospholipid barrier), epithelial (a continuous layer of surface epithelial cells interconnected by tight junctions that regulate and secrete bicarbonate, mucus, phospholipids, trefoil peptides, prostaglandins, and heat shock proteins), and post-epithelial (continuous blood flow through mucosal microvessels lined with endothelial cells forming an endothelial “barrier,” sensory nerves releasing prostaglandins, nitric oxide, and calcitonin gene-related peptide that regulates mucosal blood flow). The continuity of the epithelial cell layer renewal is preserved by the proliferation of progenitor cells, descendants of stem cells, regulated by growth factors, prostaglandin E2, and survivin, an anti-apoptotic and mitosis-promoting protein. When the mucosal barrier is affected, a cascade of pathological events appears to contribute to further damage to the mucosal layer.[21] Therefore, our finding of ghrelin revealed this sequence due to the slowing of gastric cell turnover with apoptosis occurring toward the gastric pit. The variation in ghrelin content in immunopositive cells from low to high levels could be part of the regulation of gastric gland growth in addition to orexigenic responses.[22,23] Cell proliferation increases with the growing period of age. We observed that the ghrelin immunopositive cells changed on day 28 parallel to this scenario, and it might embody another component in the complex developmental machinery. Despite the lower number of ghrelin-immunopositive cells in the EXP group than that in the CON group, there was no significant change in the number of GHSR immunopositive cells. It takes a few days for the turnover of gastric pit; however, in general, the turnover rate of G-protein coupled receptors, such as GHSR, may take several weeks. A possible reason for the discrepancy in the results between ghelin and GHSR immunopositive cells was the difference in turnover. Therefore, it is necessary to verify the changes over a longer period.
This study raised concerns about growth, cognition, and memory in the growing stage in terms of occlusal deficiency. Our study has limitations regarding the extent to which these findings are related to humans. Although ghrelin in rats resembles the same amino-peptide chain as humans, few anatomical similarities are observed between humans and rats. Other functional parameters, including serum ghrelin, intake of daily food, and vagal nerve afferents, were not evaluated in this study.
CONCLUSION
These findings suggest that BMME may trigger mucosal changes in the stomach and alter digestive function through ghrelin expression in rats. We are the first to report that occlusal change could alter ghrelin expression in the mucosa of the rat stomach, thus raising concerns about the consequential role of ghrelin in growth, cognition, and memory.
Acknowledgments
The authors acknowledge the facility and scientific and technical assistance provided by the TMDU. The authors also thank Professor Shunichi Shibata (Maxillofacial Anatomy, TMDU, Tokyo) for providing useful knowledge and guidance.
Data availability statement
The datasets generated during the current study are available upon reasonable request.
Author contributions
SMS contributed to the design of the study, animal handling, data acquisition, data interpretation, statistical analysis, and drafted the manuscript. IW contributed to the design of the study, data interpretation, and manuscript formatting. ES conceived the study. TO participated in manuscript design and formatting. All authors read and approved the final manuscript.
Ethical approval
All EXP procedures were approved by the Institutional Animal Care and Use Committee (authorization number: A2020–069A) and performed in accordance with the Animal Care Standards of TMDU.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Grants-in-Aid for Scientific Research in Japan (KAKEN) (grant numbers 20K10200 and 16K11781).
Conflicts of interest
There are no conflicts of interest.
References
- Ghrelin: Much more than a hunger hormone. Curr Opin Clin Nutr Metab Care. 2013;16:619.
- [CrossRef] [PubMed] [Google Scholar]
- The neurocognitive effects of ghrelin-induced signaling on the hippocampus: A promising approach to Alzheimer's disease. Cureus. 2018;10:e3285.
- [CrossRef] [Google Scholar]
- Ghrelin-producing cells exist as two types of cells, closed-and opened-type cells, in the rat gastrointestinal tract. Peptides. 2002;23:531-6.
- [CrossRef] [Google Scholar]
- Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-60.
- [CrossRef] [PubMed] [Google Scholar]
- Ghrelin and GHS-R in the rat gastric mucosa: Are they involved in regulation of growth during early weaning? Nutrition. 2016;32:101-7.
- [CrossRef] [PubMed] [Google Scholar]
- The influence of malocclusion on masticatory performance. A systematic review. Angle Orthod. 2010;80:981-7.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of impaired mastication on nutrition. J Prosthet Dent. 2002;87:667-73.
- [CrossRef] [PubMed] [Google Scholar]
- Unilateral maxillary molar extraction influences AQP5 expression and distribution in the rat submandibular salivary gland. Arch Oral Biol. 2012;57:877-83.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of microRNA-1, microRNA-133a and Hand2 protein in cultured embryonic rat cardiomyocytes. In Vitro Cell Dev Biol Anim. 2014;50:700-6.
- [CrossRef] [PubMed] [Google Scholar]
- Localization of motilin-immunopositive cells in the rat intestine by light microscopic immunocytochemistry. Peptides. 1994;15:987-91.
- [CrossRef] [Google Scholar]
- Occlusional modifications reversibly alter aquaporin 5 expression and localization in rat salivary glands. Front Physiol. 2020;11:528.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of chewing on appetite, food intake and gut hormones: A systematic review and meta-analysis. Physiol Behav. 2015;151:88-96.
- [CrossRef] [PubMed] [Google Scholar]
- Alimentary system and the peritoneum, omentum, mesentery, and peritoneal cavity In: Pathologic Basis of Veterinary Disease. Amsterdam, Netherlands: Elsevier; 2017. p. :324.
- [CrossRef] [Google Scholar]
- Age-related changes in haematological parameters and biochemical markers of healing in the stomach of rats with acetic acid induced injury. Toxicol Rep. 2020;7:1272-81.
- [CrossRef] [PubMed] [Google Scholar]
- Connection between masticatory efficiency and pathomorphologic changes in gastric mucosa. Quintessence Int. 2007;38:31-7.
- [Google Scholar]
- Influence of mastication on gastric emptying. J Dent Res. 2002;81:179-81.
- [CrossRef] [PubMed] [Google Scholar]
- The implication of alterations in leukocyte subset counts on immune function. Exerc Immunol Rev. 2006;12:54-71.
- [Google Scholar]
- Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255-61.
- [CrossRef] [PubMed] [Google Scholar]
- The distribution of cellular turnover in the human body. NatMed. 2021;27:45-8.
- [CrossRef] [PubMed] [Google Scholar]
- Novel approach to gastric mucosal defect repair using fresh amniotic membrane allograft in dogs (experimental study) Stem Cell Res Ther. 2017;8:1-12.
- [CrossRef] [PubMed] [Google Scholar]
- A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714-9.
- [CrossRef] [PubMed] [Google Scholar]






