Translate this page into:
Gastric emptying rate of a solid meal in patients with anterior open bite malocclusion: A preliminary study

-
Received: ,
Accepted: ,
How to cite this article: Kamaratih A, Ohmori H, Aoyagi M, Kanno Z, Ono T. Gastric emptying rate of a solid meal in patients with anterior open bite malocclusion: A preliminary study. APOS Trends Orthod 2022;12:77-85.
Abstract
Objectives:
The objective of the study was to investigate the relationship between anterior open bite (AOB) malocclusion and digestion by evaluating mastication and gastric emptying (GE) of a solid meal.
Material and Methods:
We recruited 26 female participants and divided them into two groups according to their occlusion status: The control group with normal occlusion (n = 11; age: 25.2 ± 2.8 years; and body mass index [BMI]: 21.1 ± 1.9 kg/m2) and the AOB group with AOB malocclusion (n = 15; age: 23.2 ± 5.5 years; BMI: 21.0 ± 1.6 kg/m2). GE, chewing, and occlusion were assessed simultaneously. A food questionnaire survey was also administered on the same day.
Results:
A significant positive correlation was noted between open bite and the occlusal analysis findings. Negative correlations were found between the food questionnaire score and both the open bite and occlusal analysis findings, which confirmed that AOB affects mastication. However, no significant difference in the GE rate parameters was observed between the two groups.
Conclusion:
Adaptive mechanisms may have a compensatory effect on the GE rate. However, the lack of tooth contact in the anterior occlusal region resulted in reduced masticatory ability. Orthodontic treatment should thus be considered, regardless of the presence of digestive problems, to improve mastication.
Keywords
Malocclusion
Open bite
Mastication
Breath tests
Gastric emptying
INTRODUCTION
Misalignment of teeth in patients with malocclusion affects the quality of life. The discomfort in such patients involves the psychological aspects, and overall oral health and function.[1,2] Patients who require orthodontic treatment have decreased masticatory function.[3] Among the types of malocclusion, open bite malocclusion has the most deleterious effect on masticatory performance as the number of occlusal contacts determines masticatory function.[4-6]
Anterior open bite (AOB) malocclusion is a type of malocclusion defined by the lack of contact between the anterior teeth when the jaw is in maximum closure.[7] The cause of AOB malocclusion is multifactorial and includes nasal obstruction, neuromuscular deficiency, vertical growth pattern, and poor oral habits.[8]
The correlation between malocclusion and gastric emptying (GE) rate has been previously investigated for liquid meals; however, the type of malocclusion was not specified.[9,10] The previous study confirmed that a liquid meal and a solid meal are processed through different mechanisms involving different parts of the stomach.[11] Since the effectiveness of mastication can only be observed when chewing a solid meal, a solid meal is a better option to study mastication and its effect on the part of the stomach that may get affected by the product of the mastication. To the best of our knowledge, no study has assessed the relationship between the specific types of malocclusions and GE rate using a solid meal. Therefore, this prospective study aimed to investigate the effect of AOB malocclusion on the GE rate of a solid meal.
MATERIAL AND METHODS
Ethical approval
All participants received a detailed explanation of the study objectives and methods before signing an informed consent form. The study protocol was approved by the institutional ethics committee (approval number D2018-037), and the study was conducted in accordance with the Declaration of Helsinki (2013 revision).
Study participants
We selected 15 Japanese women, who visited our clinic for orthodontic treatment of malocclusion between April 2019 and October 2020; these patients comprised the AOB group. All patients had a chief complaint of AOB with an overbite of < 0 mm requiring orthodontic treatment. Patients with any of the following were excluded from the study: A cleft lip or palate or other craniofacial syndromes; a history of abdominal surgery; a history of medication use, including gastrointestinal prokinetic agents, calcium antagonists, and selective serotonin reuptake inhibitors; pre-existing disease; habitual heavy smoking or alcohol consumption; or current or possible pregnancy. Using the same criteria for exclusion, 11 women without AOB malocclusion were recruited from among the students and staff of our clinic as the control group. The control group participants had normal occlusion and no complaints related to oral function. Both groups had no systemic condition that could have affected the study results.
Studies have shown that oral processing behavior, maximum bite force (MBF), and GE rates differ between sexes and that GE rates also depend on menstrual status in females.[12-14] Hence, female patients were exclusively selected in this study. All participants were instructed to perform the GE test in the follicular period to control for the influence of female hormones and confirmed the menstrual status before conducting the test.
Parameters
Chewing observation
All participants were instructed to ingest a 350-kcal muffin test meal (Test Meal Mix; Metabolic Solutions, Inc., Nashua, USA) comprising 90 g of dry ingredients and 80 mL of water. It was prepared in a microwave at 500 W for 3 min and 40 s and served with 150 mL water on the test day. The participants were instructed to eat the meal naturally within 15 min and to raise their hands once they had finished consuming both the muffin test meal and the water served. The number of chews and chewing time were counted and recorded manually by an observer.[5]
GE rate test
Breath test using stable isotope 13C tracers is a safe, reliable, and easy-to-perform method to measure GE rate in clinical practice.[10,15] Therefore, GE was assessed in the morning after an overnight fast using the 13C-octanoate breath test, with a validated solid muffin test meal with 100 mg 13C-labeled sodium octanoate (1-13C-sodium octanoate; Metabolic Solutions). Breath samples were collected before ingestion of the muffin test meal and at 15-min intervals for 4 h after meal ingestion using special sampling bags (Otsuka Pharmaceuticals, Tokyo, Japan). An infrared spectrophotometer (POCone; Fukuda Denshi, Tokyo, Japan) was used to measure the concentration of 13C in the breath sampling bags. The percentage of tracer detected in each bag per hour was used to calculate Tlag (time required for the stomach to reach the peak emptying rate) and T1/2 (time required to empty half the contents of the stomach) — the most common parameters used to measure the GE rate. Both parameters were calculated as previously described.[15,16]
Occlusal analysis
The occlusal contact area and MBF were measured to evaluate masticatory function indirectly. A pressure-sensitive sheet (Dental Prescale 50H type R, Fuji Photo Film Co., Tokyo, Japan) and the Occluzer FPD-709 dental occlusion pressure measurement device (Fuji Photo Film Co.) were used. Participants were instructed to sit upright, with the horizontal planes of their heads parallel to the floor. After ensuring the correct positioning of the dental prescale sheet, the participants were instructed to bite down as hard as they could for 5 s. The test was repeated 3 times with at least 30-min intervals between the tests to avoid muscle fatigue.[6]
Food questionnaire
To determine the subjective masticatory ability, a food questionnaire survey was administered. The participants were expected to be familiar with the types of food listed in the questionnaire to minimize differences in the recognition of each food.[17] Therefore, the questionnaire used in this study included questions on 55 most commonly consumed foods in Japan [Table 1]. Participants were instructed to classify each food based on the following scale: (1) can be eaten and easily chewed; (2) can be eaten with difficulty; (3) cannot be eaten as it is too difficult to chew; (4) cannot be eaten due to preference or allergy; or (5) has never been eaten. The number of food items scored as (2) or (3) was calculated using the method described previously.[18,19]
| Cooked rice | Cheese |
| White bread | Eggplant (boiled) |
| French bread | Cucumber |
| Soba noodle | Cabbage (raw) |
| Rice cake | Cabbage (cooked) |
| Konjac | Seasoned burdock |
| Lotus root (boiled) | Sweet corn |
| Fried chicken | Carrot (boiled) |
| Pork cutlet | Carrot (raw) |
| Beefsteak | Pickled radish |
| Meatball | Chinese cabbage (boiled) |
| Beef jerky | Lettuce |
| Sausage | Spinach (boiled) |
| Squid (raw) | Mushroom |
| Squid (dried) | Bean sprouts (stir fried) |
| Fish (grilled) | Enoki mushrooms |
| Tuna (raw) | Shiitake mushrooms |
| Octopus (boiled) | Pineapple |
| Abalone | Tangerine |
| Jellyfish | Apple |
| Fish cake | Banana |
| Clam (boiled) | Rice cracker |
| Tofu | Gummy candy |
| Soybean (fermented) | Cracker |
| Soybean (cooked) | Chewing gum |
| Peanut | Custard pudding |
| Omelet | Chestnut jelly |
| Sunny-side up egg |
Statistical analysis
A two-sided Wilcoxon rank-sum test was used to compare the GE rate parameters, chewing observation, occlusal analysis, and the scores of the food questionnaire survey between the two groups. Statistical significance was set at P < 0.05. All data are presented as the mean ± standard deviation unless otherwise indicated. Correlation coefficients were analyzed using Spearman’s test. All statistical analyses were performed using JMP version 14.2.0 (SAS Institute Inc., Cary, USA).
RESULTS
Study participants
The mean age, body mass index (BMI), and overbite of the study participants are summarized in [Table 2]. The AOB group had a mean age of 23.2 ± 5.5 years and a BMI of 21.0 ± 1.6 kg/m2, whereas the control group had a mean age of 25.2 ± 2.8 years and a BMI of 21.1 ± 1.9 kg/m2.
| Group | n | Age (years) | BMI (kg/m2) | Overbite (mm) |
|---|---|---|---|---|
| Control | 11 | 25.2±2.8 | 21.1±1.9 | 2.6±1.5 |
| AOB | 15 | 23.2±5.5 | 21.0±1.6 | −2.6±1.7 |
Two-sided Wilcoxon rank-sum test. AOB: Anterior open bite; BMI: Body mass index
Chewing observation
There was no significant difference between the control and AOB groups in terms of number of chews (643.3 ± 184.3 vs. 541.6 ± 153.0, respectively) and eating duration (9 min 42 s ± 3 s vs. 12 min 21 s ± 4 s, respectively) [Figure 1].
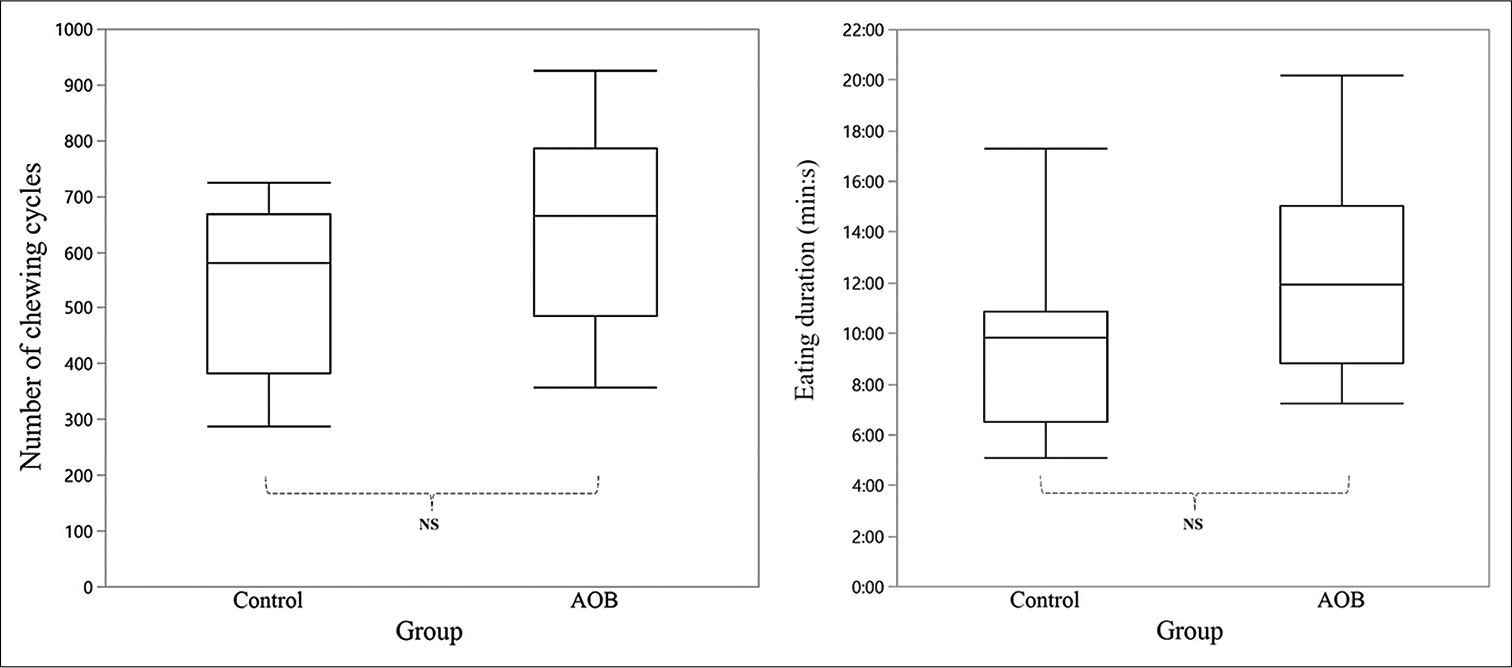
- Comparison of the chewing activities between the control (n = 11) and AOB (n = 15) groups. In the box plot, the middle line represents the median, whereas the top and bottom lines represent the first and third quartiles, respectively. Left, a number of chewing cycles is defined as the total number of chews performed to finish the muffin test meal. Right, eating duration is defined as the time required for each subject to finish the muffin test meal. AOB: Anterior open bite, NS: Not significant.
GE rate test
The 13CO2 percentage excreted in the breath per hour for both groups is shown in [Figure 2]. The curves for both groups did not show a significant difference. There was also no significant difference between the control group and the AOB group in terms of Tlag (91.1 ± 14.2 min vs. 89.9 ± 16.9 min, respectively) and T1/2 (161.9 ± 22.1 min vs. 169.3 ± 30.8 min, respectively) [Figure 3].
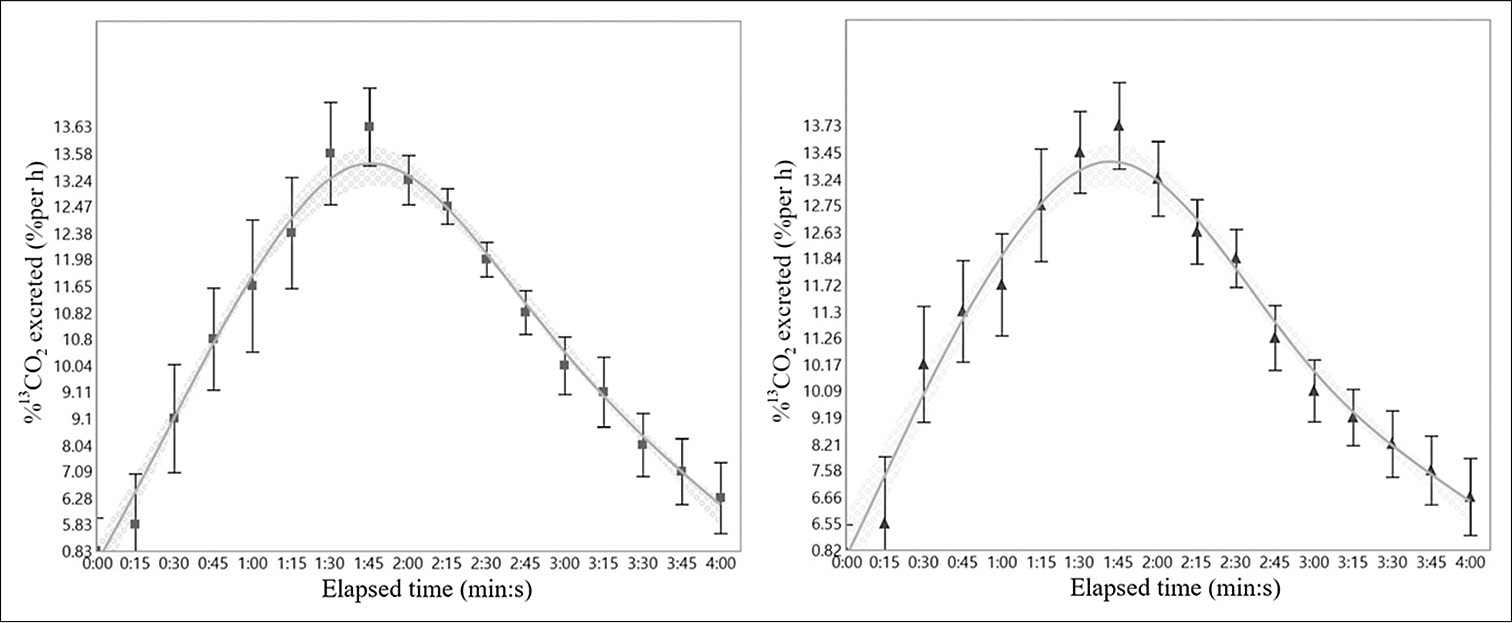
- Comparison of 13CO2 excretion per hour of the control (left, n = 11) and AOB (right, n = 15) groups. The concentration of 13C in the collected breath sampling bags was measured using an infrared spectrophotometer. Data are presented as the mean ± standard deviation.
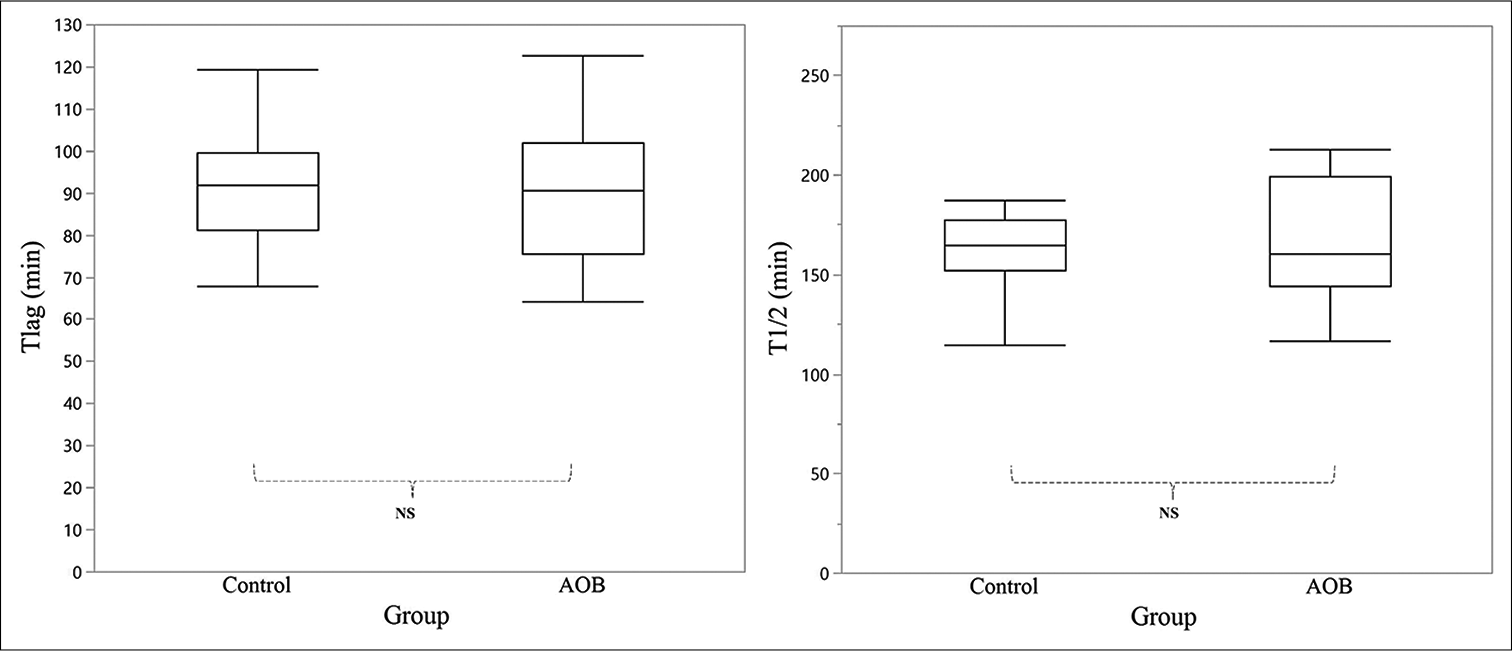
- Comparison of the gastric emptying rate parameters between the control (n = 11) and AOB (n = 15) groups. In the box plot, the middle line represents the median, whereas the top and bottom lines represent the first and third quartiles, respectively. Left, Tlag (min) is defined as the time required for the stomach to reach the peak emptying rate. Right, T1/2 (min) is defined as the time required for the stomach to empty half of its content. AOB: Anterior open bite, NS: Not significant.
Occlusal analysis
The AOB group had a significantly smaller occlusal contact area (5.3 ± 3.4 mm2) and a significantly lower maximum clenching force (175.8 ± 108.4 N) than the control group (11.5 ± 3.7 mm2; 378.5 ± 146.2 N) [Figure 4].
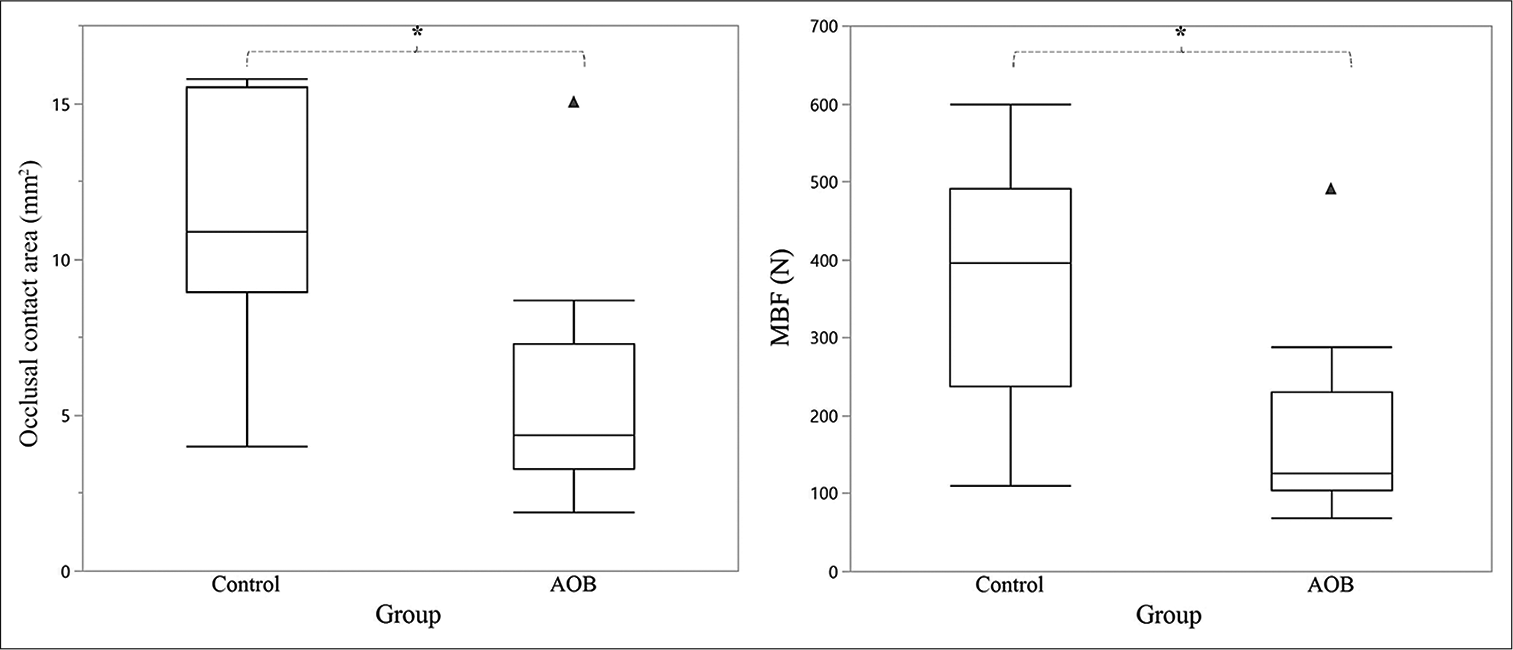
- Comparison of the occlusal parameters between the control (n = 11) and AOB (n = 15) groups. In the box plot, the middle line represents the median, whereas the top and bottom lines represent the first and third quartiles, respectively. Left, occlusal contact area (mm2) is defined as the mean occlusal contact area of each group. Right, MBF (n) is defined as the mean maximum bite force of each group. The black triangles are the outliers of the AOB group. AOB: Anterior open bite. *P < 0.05.
Food questionnaire survey
The AOB group experienced difficulty in eating significantly more types of food than the control group (7.4 ± 5.0 items vs. 0.9 ± 2.2 items, respectively) [Figure 5].
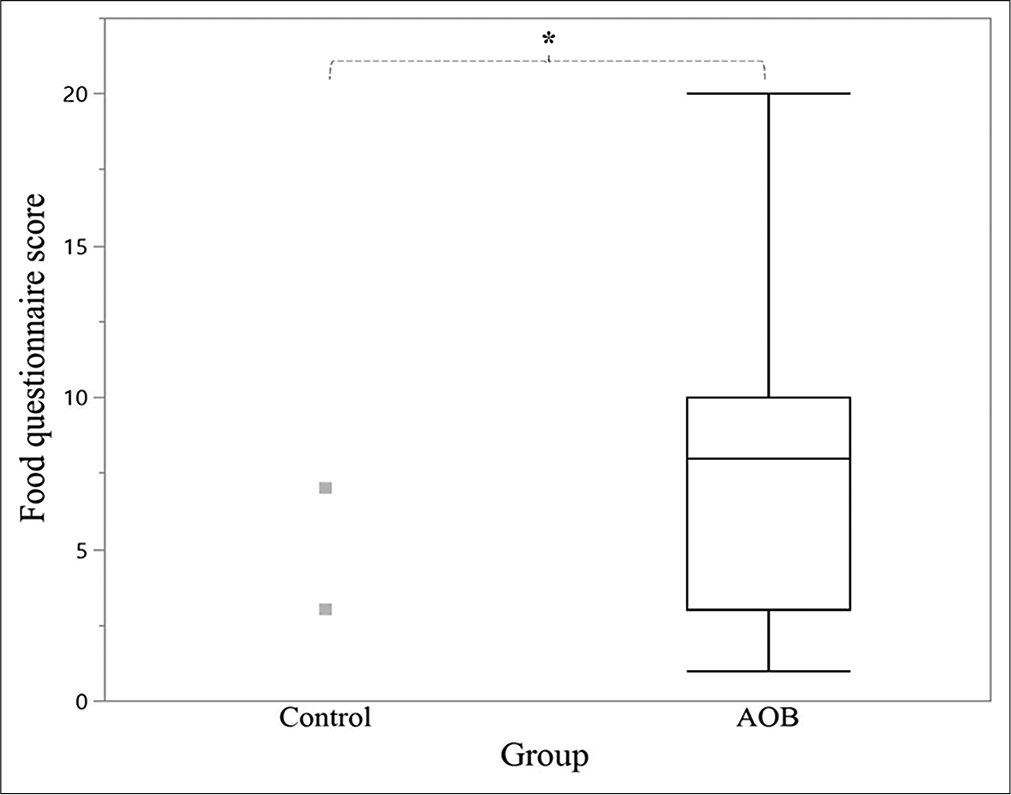
- Comparison of the food questionnaire scores between the control (n = 11) and AOB (n = 15) groups. In the box plot, the middle line represents the median, whereas the top and bottom lines represent the first and third quartiles, respectively. The food questionnaire score was calculated by adding the number of food items reported as difficult to eat by the participants in each group. The control group had a mean result of 0.9 ± 2.2 items; thus, no quartiles were visible. The gray squares are the outliers of the control group. AOB: Anterior open bite. *P < 0.05.
Correlation coefficient analysis
The results of the correlation analysis of the parameters are summarized in [Table 3]. Tlag was negatively correlated with eating duration and the total number of chews (r = −0.5). Similarly, a higher total number of chews resulted in a shorter Tlag duration. This correlation was not noted in T1/2 [Figure 6].
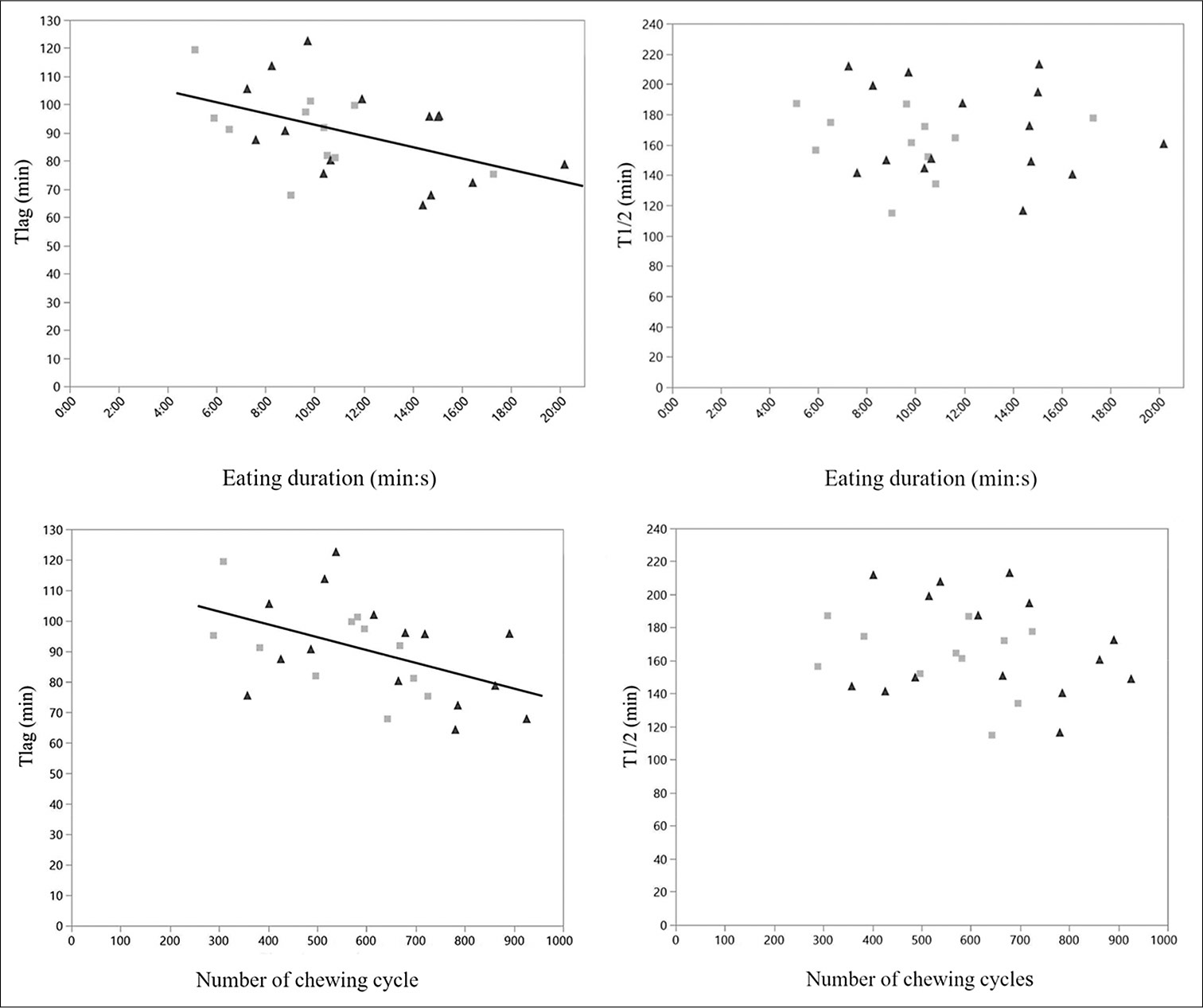
- Relationship between gastric emptying rate parameters and chewing activities. The eating duration was significantly correlated with Tlag (Top left, P = 0.0157) but not with T1/2 (Top right). The number of chewing cycles was significantly correlated with Tlag (Bottom left, P = 0.0154) but not with T1/2 (Bottom right). Gray squares depict control participants, while black triangles depict AOB participants.
| Variables | Correlation coefficient (r) | P |
|---|---|---|
| Tlag and eating duration | −0.5 | 0.0157 |
| Tlag and number of chewing cycles | −0.5 | 0.0154 |
| Overbite and occlusal contact area | 0.6 | 0.0036 |
| Overbite and MBF | 0.5 | 0.0218 |
| Overbite and questionnaire scores | −0.6 | 0.001 |
| Occlusal contact area and questionnaire scores | −0.6 | 0.0022 |
| MBF and questionnaire scores | −0.5 | 0.0087 |
Tlag, time required for the stomach to reach emptying peak (min); eating duration, total eating time (s); number of chewing cycles, total number of chewing cycles; overbite (mm); MBF: Maximum bite force; questionnaire scores, scores from the food questionnaire. Spearman’s test
A positive overbite was associated with a larger occlusal contact area (r = 0.6) and a greater bite force (r = 0.5). Moreover, a positive overbite was correlated with fewer food items perceived to be difficult to eat (r = −0.6). The same correlation coefficient results were noted between the questionnaire scores and occlusal contact area (r = −0.6) and MBF (r = −0.5).
DISCUSSION
This study found no relationship between AOB malocclusion and the GE rate of a solid meal. However, we verified that AOB malocclusion resulted in a decreased occlusal contact area and MBF — determining factors for masticatory function — as well as decreased subjective masticatory ability. Therefore, our findings demonstrate the strong relationship between AOB malocclusion and decreased masticatory function.
Patients with AOB malocclusion were included in the present study because it is speculated to affect masticatory function and digestion as the periodontal mechanoreceptors that induce cephalic-vagal stimulation is located mostly in the incisal area where there is a lack of tooth contact. The lack of sensory information received from the anterior region resulted in the inability to perform an effective chewing movement.[20] In individuals with inefficient masticatory function, the size of the bolus that is swallowed without proper trituration is predicted to affect the GE rate because a longer time is required to grind and sieve the bolus in the stomach.
Appropriate selection of a test meal is crucial in the assessment of GE rate. To investigate the relationship between masticatory function and gastric function, a solid meal is preferable.[21] The muffin test meal used in this study could retain the octanoic-acid tracer in vitro and has been well correlated with the GE rate of the solid phase in the scintigraphy test — the gold standard for GE rate measurement.[22] The caloric content of the meal was also adjusted to aid better stimulate gastric motility.[23] Compared with a toast-and-egg meal which has different caloric content, the muffin test meal was easy to prepare and standardize. In addition, because it has a dry texture, eating the muffin test meal required an adequate number of chews to mix it with saliva and form the proper bolus safe for swallowing.[24]
In this study, the number of chews of each participant was not controlled. The GE rate test requires the participant to finish the muffin test meal in 15 min. A previous study noted that chewing duration increases when chewing was performed consciously.[25] We observed that some participants spent a long time finishing the meal, although there was no restriction on the number of chews. Additional instruction in the chewing test may prolong the eating time and affect the breath test outcome. Limiting the chewing number or duration may also affect the bolus size.[26] By focusing on the effect of habitual chewing on the gastric function of patients with malocclusion, this study found a correlation between chewing behavior and GE parameters.
The results of correlation analysis showed that the longer the time spent eating a meal, the faster the attainment of the GE peak. Regardless of malocclusion, inadequate chewing time might result in swallowing larger boluses. The time needed to process such boluses subsequently results in a longer lag time. Moreover, chewing motion can activate gastric motility,[21] and the periodontal mechanoreceptor can induce cephalic-vagal stimulation and affect the antral movement.[27] Although participants with AOB lack contact between the anterior teeth in the upper and lower jaws, the mechanoreceptor in the posterior teeth and a palatable meal may have been adequate to induce cephalic stimulation. Considering the positive correlation between eating duration and a number of chews, we demonstrated that a longer chewing duration and a higher number of chews facilitated the digestion of the bolus. The lack of correlation between T1/2 and eating duration and number of chews suggests a potential adaptation in the duodenal feedback mechanism working in both fast- and slow-ingesting participants, as previously stated.[28] Another study on GE training speculated that 3 days of diet manipulation can sufficiently induce a desirable adaptive mechanism.[29] Because the participants in the AOB group had malocclusion for years, their digestive system may have been adapted to their individual eating habits.
A change in the rate of gastrointestinal movement was noted when participants performed sham feeding.[30] Moreover, sham feeding has a greater effect on the GE of a solid meal than that of a liquid meal.[31] Because of limited time, all tests were conducted on the same day. Hence, instead of conducting a masticatory test that requires chewing in the middle of the breath test, the food questionnaire survey and occlusal analysis were performed.
The study participants in the AOB group reported difficulty in chewing many types of foods, whereas those in the control group reportedly experienced chewing difficulty with fewer types of foods. The questionnaire score was higher for those with a more severe open bite. These results are consistent with those of a previous study in which patients with malocclusion had a higher questionnaire score than the control participants.[5] There was also a report that females showed a higher association of open bite condition to oral health-related quality of life more than the male participants.[2] In another study, it was revealed that in females, AOB mostly affected subjective functional limitation.[32] A study comparing questionnaire scores before and after orthodontic treatment found a significant improvement in subjective masticatory ability.[19] Since the subjective perception of mastication ability affects a person’s quality of life more than the actual condition of the mouth,[33] this result emphasizes the importance of treating severe AOB.
The AOB group had a smaller occlusal contact area and MBF than the control group. Both parameters of the occlusal analysis test also correlated positively with the severity of the open bite. The more severe the open bite condition, the lower the occlusal contact area and MBF values. These results are similar to those of a previous study, which reported that the largest occlusal contact area is observed in participants with normal occlusion and that participants with open bite have the lowest occlusal contact areas among those with other types of malocclusions.[6] A patient with an open bite generally has less MBF because of the unbalanced relationship between the occlusal plane and masseter muscles.[7,34] The previous study examining the EMG activity of the masseter muscle and anterior temporalis muscle of the patients with AOB showed lower peak amplitude compared to those with normal occlusion. The different percentage of muscle fiber and inefficient chewing pattern caused by lack of input from mechanoreceptors in the anterior region was suggested to yield the difference.[20] In this study, the AOB group showed a lower MBF than the control group. Correlations between both the occlusal contact area and MBF and the questionnaire scores were also noted, demonstrating that a reduced occlusal contact area or MBF resulted in decreased self-assessed masticatory ability.
This study had several limitations. First, chewing was assessed by direct observation, with manual counting of the number of cycles and chewing duration. Because of strict regulations against the facial recording of the participants, digital data could not be used to confirm the researcher’s observations. In the future, an electromyography test should be considered to use to measure jaw movement while chewing a solid meal.[34] Second, due to the long hours of observation required to conduct the study and the strict regulation from the ethics committee of our hospital, it was necessary to minimize the number of study participants. To confirm the reliability of our results, we performed a power analysis using the G*Power 3.1.0 software package (Universität Düsseldorf, Düsseldorf, Germany).[35] The effect size (Cohen’s d) was obtained by referring to a similar study conducted previously.[9] We used an α = 0.05 error probability (critical t: 2.1088892; noncentrality parameter δ: 2.9934343) and 80% power (actual power, 0.8056306). The results suggested that a total of 20 participants would be sufficient to achieve significant results. Third, to control the influence of female hormones on the GE rate and the potential difference in chewing observation and occlusal analysis between sexes, we only recruited women in the follicular period.[12–14] Although these steps were taken to minimize the heterogeneity of our small sample size, this study’s results cannot be generalized to male subjects. Future studies should include both male and female participants to observe the influence of sex-specific factors on the GE rate in patients with AOB malocclusion. Although not significant, severe open bite showed a potential correlation with both parameters of the GE rate. Future studies should consider recruiting only patients with severe cases of malocclusion. Furthermore, AOB has multifactorial etiologies such as nasal obstruction, neuromuscular, facial growth, and oral habits.[8] The future study should consider the possibility of the different effects caused by different etiology to the masticatory function as well as gastric function. To properly measure the masticatory function, an effective method should be developed to elucidate the overall function of the digestive system.
CONCLUSION
No relationship was found between AOB malocclusion and GE rate in the digestion of the test meal. The lack of tooth contact in the anterior occlusal region resulted in reduced masticatory ability. Although the participants did not report digestive problems, the low masticatory ability score of the various types of food in the questionnaire indicates that the quality of life of patients with AOB malocclusion is affected. Therefore, orthodontic treatment should be considered in such patients to improve masticatory function.
Our findings highlight the importance of using a solid meal in studies that evaluate the relationship between malocclusion and gastric function. A more appropriate solid meal that can be used to assess both masticatory function and GE rate, which also involves a simple preparation process, should be developed.
Acknowledgment
The authors would like to thank Dr. Masako Akiyama for providing statistical analysis support. The abstract of this research had been presented at the 78th Japan Orthodontic Society Conference and 9th International Orthodontic Congress.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Ministry of Education, Culture, Sports, Science, and Technology in Japan 18K09761, 18K09853, 20K18750, 21K10181.
Conflicts of interest
There are no conflicts of interest.
References
- Effect of malocclusion severity on oral health related quality of life in Malay adolescents. Health Qual Life Outcomes. 2021;19:71.
- [CrossRef] [PubMed] [Google Scholar]
- Gender-specific associations of malocclusion traits with oral health-related quality of life in a Finnish adult population. Eur J Orthod. 2020;42:242-9.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between orthodontic anomalies and masticatory function in adults. Am J Orthod Dentofac Orthop. 2007;131:216-22.
- [CrossRef] [PubMed] [Google Scholar]
- Masticatory function in patients with dentofacial deformities before and after orthognathic treatment a prospective, longitudinal, and controlled study. Eur J Orthod. 2015;37:67-72.
- [CrossRef] [PubMed] [Google Scholar]
- Application of “prescale” as an aid to clinical diagnosis in orthodontics. Bull Tokyo Dent Coll. 2004;45:99-108.
- [CrossRef] [PubMed] [Google Scholar]
- Masticatory evaluation of anterior open bite malocclusion using the colorimetric capsule method. Gen Dent. 2018;66:56-9.
- [Google Scholar]
- Anterior open bite and its relationship with dental arch dimensions and tongue position during swallowing and phonation in individuals aged 8-16 years: A retrospective case-control study. Int Orthod. 2021;19:107-16.
- [CrossRef] [PubMed] [Google Scholar]
- Gastric emptying rate in subjects with malocclusion examined by [(13) C] breath test. J Oral Rehabil. 2013;40:574-81.
- [CrossRef] [PubMed] [Google Scholar]
- Gastric emptying rate before and after orthodontic treatment examined with the [(13) C] breath test: A pilot study. Am J Orthod Dentofac Orthop. 2018;153:347-54.
- [CrossRef] [PubMed] [Google Scholar]
- Disintegration of solid foods in human stomach. J Food Sci. 2008;73:R67-80.
- [CrossRef] [PubMed] [Google Scholar]
- Normal solid gastric emptying values measured by scintigraphy using asian-style meal: A multicenter study in healthy volunteers. J Neurogastroenterol Motil. 2014;20:371-8.
- [CrossRef] [PubMed] [Google Scholar]
- Relating oral physiology and anatomy of consumers varying in age, gender and ethnicity to food oral processing behavior. Physiol Behav. 2020;215:112766.
- [CrossRef] [PubMed] [Google Scholar]
- Masticatory efficiency and bite force in individuals with normal occlusion. Arch Oral Biol. 2014;59:1065-74.
- [CrossRef] [PubMed] [Google Scholar]
- Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104:1640-7.
- [CrossRef] [Google Scholar]
- Wagner-Nelson method for analysing the atypical double-peaked [13CO2] excretion curve in the [13C]-octanoate gastric emptying breath test in humans. Clin Exp Pharmacol Physiol. 2005;32:590-4.
- [CrossRef] [PubMed] [Google Scholar]
- Consensus on the terminologies and methodologies for masticatory assessment. J Oral Rehabil. 2021;48:745-61.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between occlusal condition and the changes of bolus property during mastication. Kokubyo Gakkai Zasshi. 2006;73:107-15.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of orthodontic treatment on occlusal condition and masticatory function. Bull Tokyo Dent Coll. 2014;55:185-97.
- [CrossRef] [PubMed] [Google Scholar]
- Chewing pattern and muscular activation in open bite patients. J Electromyogr Kinesiol. 2012;22:273-9.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of mastication on gastric emptying. J Dent Res. 2002;81:179-81.
- [CrossRef] [PubMed] [Google Scholar]
- Simultaneous measurement of gastric emptying with a simple muffin meal using [13C]octanoate breath test and scintigraphy in normal subjects and patients with dyspeptic symptoms. Dig Dis Sci. 2002;47:1657-63.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of meal size and test duration on gastric emptying and gastric myoelectrical activity as determined with simultaneous [13C] octanoate breath test and electrogastrography in normal subjects using a muffin meal. Dig Dis Sci. 2001;46:2643-50.
- [CrossRef] [PubMed] [Google Scholar]
- Chewing behavior and salivary secretion. Eur J Oral Sci. 2004;112:19-24.
- [CrossRef] [PubMed] [Google Scholar]
- Volitional chewing with a conscious effort alters and facilitates swallowing during feeding sequence. J Oral Rehabil. 2014;41:191-8.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of the relationship between masticatory performance, occlusal contact area, chewing time and cycles, and gastric emptying scintigraphy in dentate subjects. J Oral Rehabil. 2019;46:787-91.
- [CrossRef] [PubMed] [Google Scholar]
- Gastric emptying rate in subjects with experimentally shortened dental arches: A pilot study. J Oral Rehabil. 2008;35:402-7.
- [CrossRef] [PubMed] [Google Scholar]
- Insulin and incretin hormone responses to rapid versus slow ingestion of a standardized solid breakfast in healthy subjects. Endocrinol Diabetes Metab. 2019;2:e00056.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of improved use of chewing gum during capsule endoscopy in decreasing gastric transit time: A prospective randomized controlled study. Front Med. 2021;8:605393.
- [CrossRef] [PubMed] [Google Scholar]
- Sham feeding cephalic-vagal influences on gastric myoelectric activity. Dig Dis Sci. 1989;34:521-7.
- [CrossRef] [PubMed] [Google Scholar]
- Oral-health-related quality of life (OHRQoL) and anterior open bite in adult patients: A case-control study. Healthc. 2022;10:129.
- [CrossRef] [PubMed] [Google Scholar]
- Subjective food intake ability in relation to maximal bite force among Korean adults. J Oral Rehabil. 2009;36:168-75.
- [CrossRef] [PubMed] [Google Scholar]
- Decreased chewing activity during mouth breathing. J Oral Rehabil. 2012;39:559-67.
- [CrossRef] [PubMed] [Google Scholar]
- G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175-91.
- [CrossRef] [PubMed] [Google Scholar]






